Fig. 6.
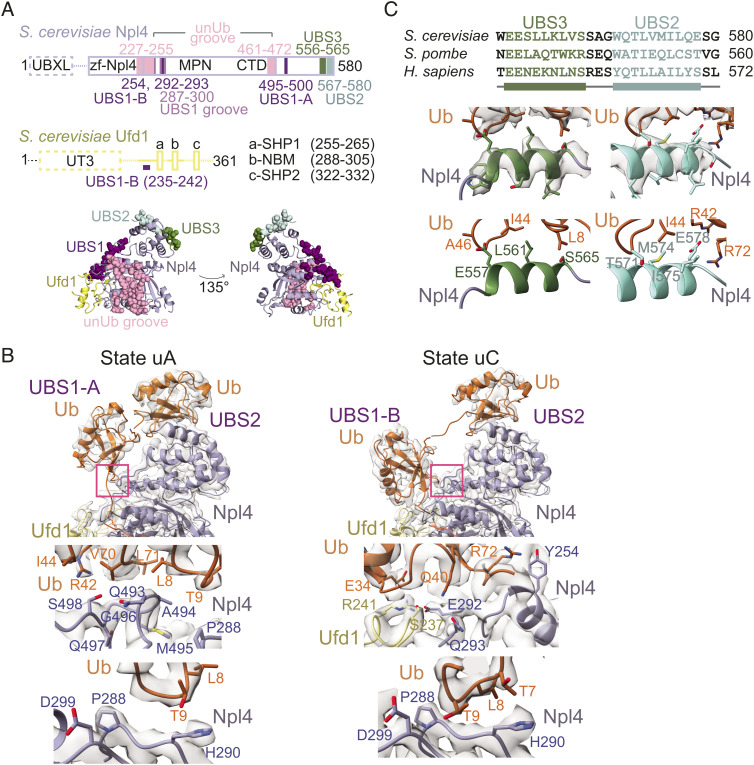
Sites of ubiquitin interaction in Ufd1/Npl4/Cdc48. (A) Ufd1/Npl4 domain organization. Solid lines indicate regions modeled in cryo-EM reconstructions. The four sites of ubiquitin association are colored and residues of the sites are shown as spheres in the cartoon representation below. Ufd1 is in yellow and Npl4 is in light blue. (B) Top: Close-up of ubiquitin in UBS1 in EM density maps and models of states uA and uC. uA represents states where ubiquitin occupies UBS1-A (also observed in states intA and intB) while uC represents states where ubiquitin occupies UBS1-B (also observed in uD). Pink boxes highlight sites of ubiquitin interaction shown in Lower panels. Middle Left: Interactions between ubiquitin in UBS1-A and Npl4. Side chains of residues in ubiquitin and Npl4 that are positioned to mediate the association are shown and labeled. Middle Right: Interactions between ubiquitin in UBS1-B and Ufd1/Npl4. Side chains of residues in ubiquitin, Npl4 and Ufd1 that mediate contacts are shown and labeled. Bottom: The Npl4 pivot groove. Positioning of the β1–β2 loop of ubiquitin at the Npl4 pivot groove in UBS1-A (Left) and UBS1-B (Right) are shown. (C) EM density map and models representing UBS3 and UBS2 as derived from state uD, and their sequence alignment. UBS3 and UBS2 include two helices separated by a short linker that interact with tandem ubiquitin molecules in the Lys48 chain.