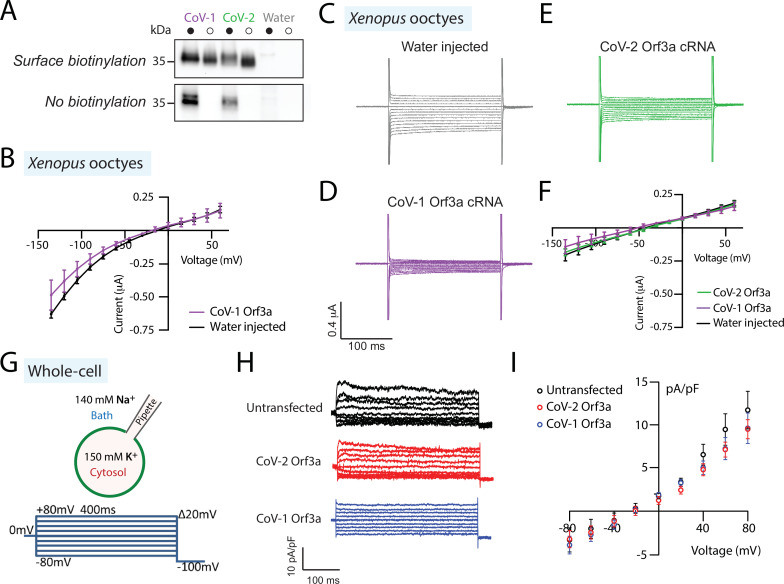
Figure 2. SARS-CoV-2 Orf3a is not a viroporin.
(A–C) SARS-CoV-2 (CoV-2) Orf3a does not elicit a cation current at the plasma membrane. (A) Solutions used for whole-cell patch-clamp experiments. (B) I-V relationship for HEK293 cells expressing CoV-2 Orf3aSNAP by doxycycline induction in various external cationic solutions (Na+, n=26; K+, n=5; Cs+, n=8; NMDG+, n=8; Ca2+, n=5). Mean traces are colored based on Figure 2A. (C) Average current density for untransfected HEK293 cells (gray bars) and cells transfected with CoV-2 Orf3aSNAP (red bars) at –80 and +80 mV recorded in Na+ (n=11), K+ (n=8), and Cs+ (n=8) solutions. (D–I) CoV-2 Orf3a does not elicit a Na+, K+, or Ca2+-selective current in endolysosomes. (D, G) Solutions used in the endolysosomal patch-clamp experiments. All the bath solutions contained 150 mM Cl- and pipette solutions contained 5 mM Cl- (E, H) I-V relationship for endolysosomes from HEK293 cells expressing GFP (control, black) or CoV-2 Orf3aHALO (green). (F, I) Average current density for control and CoV-2 Orf3aHALO expressing HEK293 cells at –120 mV and +120 mV from (D, G). (J–L) CoV-2 Orf3a does not elicit a current in Xenopus oocytes when recorded in high K+ external solution. (J–K) Representative current traces from Xenopus oocytes injected with (J) water or (K) CoV-2 Orf3a2x-STREP cRNA (20 ng). Recordings are done in high external K+ (96 mM KCl) that reproduces published methods. (L) I-V relationship for water-injected (black, n=7) or CoV-2 Orf3a (green, n=7) following protocol described in (J–K).