Background:
In breast augmentation, during submuscular or dual plane dissection, anatomical variations of the inferior and costal origin of the pectoralis major muscle (PMM) play a key role to ensure optimal implant coverage. Especially, a short and narrow muscle or surgical release along the sternum increases the risk of irregularities and animation deformities of the implant.
Methods:
In 84 consecutive aesthetic breast augmentations intraoperatively, measurement of PMM dimensions was performed bilaterally. These PMM measurements were then correlated with the preoperative breast width, the inframammary fold, and the placement of the implant’s lower pole.
Results:
One hundred sixty-eight PMMs of 84 patients were dissected with a dual plane II or III technique for primary aesthetic breast augmentation. In 88% of breasts, the calculated implants’ lower pole was below the inferiomedial origin of the pectoralis muscle. In 10% of patients, a separation (more than 1 cm wide and 2 cm wide) in the inferior-medial origin of the PMM was noted. An asymmetry more than 0.5 cm in length between the left and right pectoralis major was noted in 36% of patients.
Conclusions:
In this series, the anatomy of the PMM demonstrates a substantial variability in width and length and a considerable asymmetry in its dimensions. These findings emphasize the importance of good access and visualization of the origin of the PMM fibers before its division.
Takeaways
Question: The presented study aimed to show the importance of anatomical variations of the pectoralis major muscle for breast implant surgery.
Findings: In 84 aesthetic breast augmentations, measurement of pectoralis major muscle dimensions was performed bilaterally. In 88% of breasts, the calculated implants’ lower pole was below the inferiomedial origin of the pectoralis muscle. In 10% of patients, a separation (more than 1 cm wide and 2 cm wide) in the inferior-medial origin of the pectoralis major muscle was noted.
Meaning: Regarding the highly variable anatomy of the pectoralis major muscle, a clear visualization of the muscle origin is mandatory for breast augmentation surgery.
INTRODUCTION
Implant-based breast surgery is one of the most common surgical procedures in plastic surgery.1,2 The pectoralis major muscle (PMM) plays a key role in aesthetic and reconstructive procedures. If the tissue cover is thin, it can provide a soft and smooth tissue coverage among the décolletage, which reduces the implant palpability and visibility. The PMM is a fan-shaped muscle of the anterior chest wall.3 The origin of the PMM includes the anterior surface of the medial half of the clavicle, first through seventh rib cartilages, and the superior part of the external oblique aponeurosis.4 The fibers of the superior, medial, and inferior origins run obliquely to converge into a flat tendon that inserts on the greater tubercle of the humerus.3,4
The PMM also improves aesthetic appearance and decreases the risk of capsular contracture.1,5 The dual plane position with release of the inferior part of the PMM allows the lower pole of the implant to be without muscle coverage.1,6 This results in better expansion and a more natural appearance of the lower pole of the breast in addition to a minimized risk of animation deformities.6–8
Technical considerations of the dual plane dissection along with the anatomical variability of the PMM are crucial to ensure optimal aesthetic outcomes.6,9 This study evaluated anatomical variations of the PMM and further discussed technical considerations, recommendations, and implications of these findings for implant-based breast augmentation.
MATERIALS AND METHODS
The dimension of the PMM of 84 consecutive female patients was measured intraoperatively during primary implant-based breast augmentation with a dual plane II or III technique. All patients were counseled in accordance with the Declaration of Helsinki guidelines, and written informed consent was obtained preoperatively. All procedures and measurements were performed by the senior author (P.H.). The preoperative measurements included breast width (BW) and position of the existing inframammary fold (IMF). All implants were selected and preoperatively marked according to the Akademikliniken (AK) method.10
The fundamental principle of the AK method includes the positioning of the implants vertically, so that 50% of the height of an anatomical implant can be placed inferiorly to the nipple-areolar complex (NAC) postoperatively. This is in accordance with the definition of the natural aesthetic breast as described by Mallucci and Branford,11 which details that augmented breasts should have about 50% of their volume of round implants, and therefore, 55% of the height should be positioned distal to the NAC.11
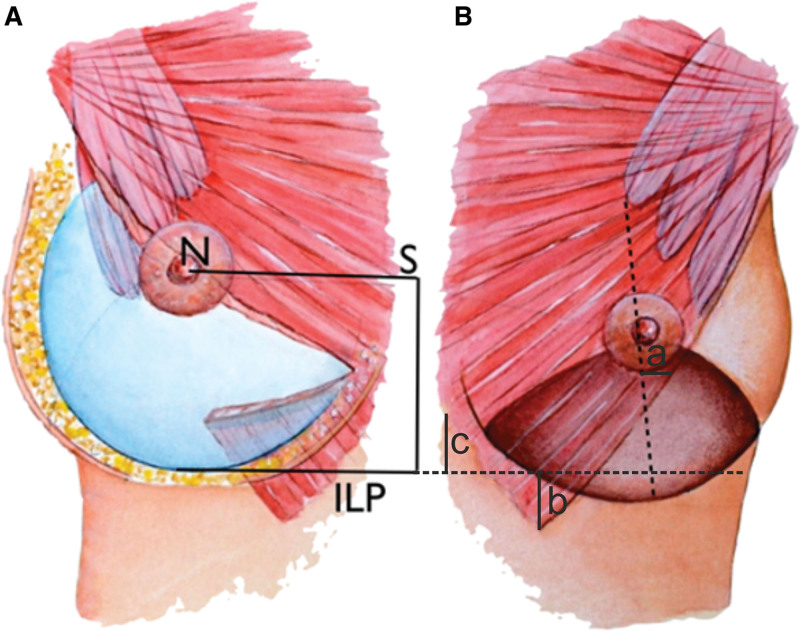
These definitions and the AK marking method are well documented and have been utilized in this study, following a vast amount of implant-based breast augmentations performed by the senior author (P.H.).2,10 Following these principles, arm elevation of 45 degrees above the horizontal plane accurately predicts the postoperative nipple elevation. This arm elevation is achieved by asking the patient to position the arms on their head and allows the nipple position to be horizontally transferred (NS line) to the midline where the skin envelope strongly adheres to the chest wall. From the midline point, a distal measurement of 50% or 55% for anatomical or round implant height, respectively, is measured. At the distal end of this midline measurement, a horizontal implant lower pole (ILP) line is marked parallel to the nipple to sternum line marking (NS) (Fig. 1A). The relationship between existing IMF and the (calculated) ILP was measured and documented. Measurements were done to evaluate variability between patients as well as to evaluate left-to-right symmetry in each individual patient. Any left-to-right measurement difference above 1 cm was considered asymmetrical.
Fig. 1.
Schematic drawing of breast implant and pectoralis major muscle dimensions. A, Elevation of the NS line after augmentation. The NS line is preoperatively marked with hands on top of the head, and the ILP line (ILP) is drawn parallel to this from a midline point at half the implant height (for anatomical implants) distal to the NS line. The PM muscle is divided 2–3 cm above the ILP line and 2–3 cm distal to the NS line in the midline, permitting retraction of the lateral part of the muscle and minimized postoperative animation. B, Intraoperative measuring points before implantation: (a) width of the PMM in relation to the breast meridian halfway between the NS and ILP lines; (b) the (vertical) distance between the inferiomedial origin of PMM and the ILP line; and (c) distance between sternal PMM origin and ILP line. The darker area illustrates the supramuscular dissection in dual plane II-III dissection.
Furthermore, intraoperative dimensions were obtained using a sterile metal ruler with millimeter scale. The breast gland density was subjectively graded and documented in all cases by the operating surgeon as well, moderately, or poorly defined.
Following, dimensions of the PMM were evaluated intraoperatively (Fig. 1B): width of PMM in relation to breast central meridian halfway between the ILP and the nipple; the (vertical) distance between the lowest fibers of PMM and the ILP line at the inferiomedial origin of the muscle and at the sternal origin; and the rib origin of the PMM and pectoralis minor muscle (Pmm). Furthermore, the width of the PMM was measured at the IMF and correlated with the BW. A PMM inferior origin split was defined as any split with a width more than 1 cm and length more than 2 cm. All present splits of the inferior PMM fibers were documented.
Statistics
Data are shown as means (range). Pearson’s χ2 test and Fisher exact test were used for categorical variables (the Fisher exact test was used instead of χ2 test when expected value of any cell was below 5). For continuous variables, independent t test was used. A P value below 0.05 was considered statistically significant.
RESULTS
Eighty-four consecutive female patients received a total of 168 implants for primary implant-based breast augmentation. All implants used were in anatomical shape and textured, with a mean volume of 287 mL (170–410 mL) (Allergan, Dublin, Ireland). The mean BW was 11.7 cm (10–13 cm). The level of the IMF was consistently originating at the level of the sixth and seventh ribs (Table 1). Out of 84 patients, 30 patients (36%) had asymmetrical (more than 0.5-cm side difference) PMM and 28 patients (33%) also had asymmetrical Pmm origin (Table 1). The calculated ILP-line position coincided (less than 1 cm) with the existing IMF in 87 (51.8%) breasts. In 75 (44.7%) breasts, the calculated ILP-line was distal (more than 1 cm) to the preoperative IMF, and in six (3.5%) breasts, the calculated ILP was proximal to the preoperatively existing IMF (Table 1). In 59 breasts (35.1%), the IMF and glandular density were well defined, in 85 (50.6%), they were moderately defined, and in 24 breasts (14.3%), they were poorly defined.
Table 1.
Level of the IMF, Asymmetries of the PMM and Pmm, Relationship of ILP to IMF, and Glandular Density
| Level of IMF | % (Patients) |
|---|---|
| Rib 5 | 3.5 (3) |
| Rib 6 | 50.6 (43) |
| Rib 7 | 45.9 (39) |
| Asymmetries of PMM | |
| 1 Rib (0.5–1 cm) | 36 (30/84) |
| 2 Ribs (1–2 cm) | 63 (19/30) |
| 3 Ribs (2–3 cm) | 20 (6/30) |
| Asymmetries of Pmm | |
| 1 Rib (0.5–1 cm) | 33 (28/84) |
| 2 Ribs (1–2 cm) | 71 (20/28) |
| 3 Ribs (2–3 cm) | 21 (6/28) |
| Relation of ILP-line to IMF | % (breasts) |
| ILP proximal to IMF | 3.5 (6) |
| ILP at IMF | 51.8 (87) |
| ILP distal to IMF | 44.7 (75) |
| Glandular density | |
| Well defined | 35 (59) |
| Moderately defined | 51 (85) |
| Poorly defined | 14 (24) |
Intraoperatively, the lateral border of the PMM halfway between the nipple and the ILP-line (Fig. 1B) was lateral to the breast meridian in 136 (81.0%) breasts, at the breast meridian (±0.5 cm) in 25 (14.9%) breasts, and medial to the meridian in seven (4.2%) breasts. In 148 (88%) breasts, the sternal border of PMM was above the calculated ILP-line, while the inferiomedial PMM origin was above the ILP-line in 37 breasts (Table 2).
Table 2.
Width of PMM in Relation to Breast Meridian and Relationship of PMM Sternal Origin Fibers and Inferiomedial PMM Origin to ILP Line
| Width of PMM Halfway between NAC and ILP in Relation to Breast Meridian | % (Breasts) |
|---|---|
| Lateral | 81 (136) |
| At | 15 (25) |
| Medial | 4 (7) |
| Relationship of Inferior PMM Sternal Fibers to ILP Line | |
| Below | 47 (79) |
| At | 16 (27) |
| Above | 37 (62) |
| Relationship of Inferiomedial PMM Origin to ILP Line | |
| Below | 1 (2) |
| At | 11 (18) |
| Above | 88 (148) |
There was no correlation between the preoperatively measured BW and preoperatively measured width of the underlying PMM at the IMF level.
DISCUSSION
Although many anatomical studies have documented the relationship between the IMF and various dimensions of the PMM,4,10,12 there is a lack of objective data regarding the PMM dimensions and origin, and its relation to the ILP position. Normally, breast implants were positioned at the existing IMF. However, in cases with short lower pole breast, tuberous breast, or pseudoptotic breast, the implants have to be placed distal to the existing IMF to create an ideal aesthetic appearance.11
In about half of the analyzed breasts, the ILP was positioned (more than 1 cm) distal to the existing IMF. If the IMF and glandular tissue are poorly defined, this can usually be done without postoperative lower pole irregularities. However, if the IMF is well defined and the glandular tissue is dense, the risk of double bubble deformation is high, and additional measures, such as scoring or fat grafting, are needed to prevent these deformities. In our study, the IMF and glandular tissue were well defined in 59 breasts (35%).
Of great interest was the absent relationship between the preoperative width of the breast and the width of the PMM at the IMF level. Thus, preoperative BW does not provide any guidance about the width of the PMM. Baek et al3 measured 50 breasts and found that the PMM was attached to the sixth rib in 80% and superior to the IMF in 66.7% of cases. In 50.6% cases of our study, the muscle was attached to the sixth rib.
If a dual plane I technique is used, subglandular undermining has to be limited with respect to the findings that the PMM is often short and the potential risk that the serratus muscle is mistaken for being pectoralis major fibers. Furthermore, in dual plane I dissection, the inferiomedial PMM fibers also have limited retraction away from the implant, which increases the risk for animation deformities, according to the authors' experience. Subsequently, dual plane I was avoided in all patients in this series, and the supramuscular undermining was done to the NAC or above it, before the muscle was divided. None of the patients in this series had any pronounced animation deformity. To achieve these outcomes, it is believed that the lateral retraction of the PMM from the capsule and maintaining of the PMM origin 2–3 cm distal to the NS line in the midline is of great importance.
Sim et al13 also evaluated the anatomy and tensile strength of the abdominal head of the PMM in relation to transaxillary breast augmentation in a study of 12 cadavers and in 92 intraoperative cases. They found the abdominal head of the PMM to be too adherent to be dissected with blunt force, further underlining the importance of direct visualization for adequate muscle division.
In 16% of breasts, the inferiomedial border of PMM coincided with the calculated level of the ILP (Table 2). In 47.0%, the inferiomedial PMM border extended distal to the ILP. These findings underline the importance of visualizing the inferior origin of the PMM during axillary implantation. If these procedures are done blindly and these long muscles are not visualized and divided, it is likely that they will result in more animation deformities.
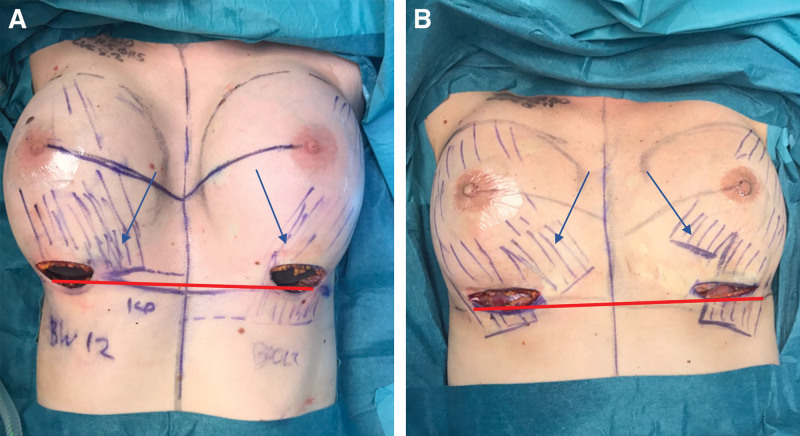
In our study, 10% of patients had a separation in the inferior origin of the PMM, which can result in animation deformities. If the lateral and long slip of the PMM is not divided well above the ILP line, the implant may herniate through the muscle separation during PMM tightening (Fig. 2).14,15
Fig. 2.
Intraoperative view of anatomical PMM variations after the breast implant inset. A, Asymmetry of PMM between left and right side, with ILP line below PMM origin on right side and above on left side. B, Medial split of PMM fibers on both sides. Red bar indicates ILP line. Blue arrows indicate PMM fibers.
When the PMM is very short, it cannot be incised at the level of the IMF as recommended by Tebbetts.1 In describing and discussing the PMM division in dual plane implants positioning, the relationship between the implant and the preexisting IMF is a frequent debate. If the PMM is divided at this ILP line, it makes recreation of a new IMF very difficult as a robust suture fixation between the Scarpa fascia and the thoracic fascia16 is ruined by the muscle division. To provide a stable fixation and recreation of the IMF in this case series, the PMM was divided 2–3 cm above the ILP-line. It is important to know that halfway between the nipple and the ILP-line, where the width of the muscle was measured, 81% of breasts had a muscle that extended lateral to the breast meridian, but medially in only 4%. When dividing the muscle close to this level, it is important that the surgeon is aware of this great variability in lateral muscle position and the relation to the implant inserted.
Madsen et al17 argued that PMM needs to be at least 80% of BW to provide adequate implant coverage and avoid window shading, otherwise an acellular dermal matrix may be needed.18 However, they did not specify the level at which PMM width was measured and how it influenced their technique. In this present study, the PMM was narrow in 19% of the patients (at the NAC or medial to it halfway in between the NAC and the ILP line). Despite this, no patients were found to have poor muscle coverage and in no case was there a need for use of ADM to provide an adequate coverage of the implant. There is no doubt that the most important muscle coverage is in the cleavage and along the sternum, and we believe that preservation of muscle 2–3 cm distal to the NS line is most important for an adequate implant coverage. To minimize animation deformities in body builders, the so-called “dual plane IV” technique is proposed. In these cases, the muscle width is narrowed by extended subglandular dissection (above the NAC) and high lateral oblique division of the muscle is performed. The origin of the PMM is still preserved here 2–3 cm distal to the NS line. This provides good coverage in the cleavage minimizing visibility and rippling but without deformities during PMM animation.
Sanchez et al12 in 101 cadaveric dissections (of both male and female subjects) found asymmetry in 10% of cases. They found that in 24% of cases, the pectoralis major and minor fibers were separated by less than 1 cm of vertical distance. This can result in inadvertent dissection below the Pmm. To avoid dissection under the Pmm, the senior author (P.H.) recommends that the steps of dissection should be proceeded as follows:
Lift the lateral border of the PMM and divide obliquely parallel to the rib cage to weaken lateral PMM fibers and minimize risk for accidental dissection into intercostal fibers.
Dissect medial in the direction of the sternal notch with electrocautery, with proactive hemostasis, directly under the PMM, leaving the loose connective tissue on the ribs.
At the upper medial border of the implant (measured with a steel ruler), the dissection is performed in a lateral direction. This will automatically enter into the space between the PMM and Pmm.
The final muscle division is at the inferiomedial PMM origin. If a finger is placed externally on the NS line in the midline, this is a useful guide in avoiding high division of the PMM along the sternum.
In view of the variability of the inferior attachments of the PMM, for example, the relatively frequent occurrence of a muscle split, visualization of the muscle is essential. The IMF incision provides the closest access and best visualization of the muscle, whereas blind axillary dissection offers no visualization and control of muscle division. Even if endoscopy is used in axillary implantation, it does not offer the same controlled definition of the IMF especially if this has to be recreated in a new position.19 The same thing applies for periareolar implantations. Furthermore, axillary implantation is very limited in different dual-plane dissection techniques, which cannot be performed appropriately. Thus, we believe that the method of choice for most breast augmentations should be an IMF approach with an exact calculation of the position of the new IMF.2,18
CONCLUSIONS
The anatomy of the PMM shows considerable variability in width and length. There is general consensus on the fact that the inferior origin of the PMM has to be divided to give good lower pole expansion and minimized animation deformity. Good knowledge about the variability of the PMM is important for accurate muscle division. This study underlines the importance of direct visualization of the PMM. This is best achieved through an inframammary approach, which gives the best control of muscle division, implant positioning, and definition of the IMF.
Footnotes
Published online 18 January 2023.
Disclosure: Dr. Montemurro is a consultant and speaker for Polytech. Dr. Hedén is a consultant and speaker for Mentor Co and Establishment Lab. He is also a shareholder in Establishment Labs and Polytech Co, and he has a royalty agreement with Allergan Co. The other authors have no financial interest to declare.
REFERENCES
- 1.Tebbetts JB. Dual plane breast augmentation: optimizing implant-soft-tissue relationships in a wide range of breast types. Plast Reconstr Surg. 2006;118:81S–98S. [DOI] [PubMed] [Google Scholar]
- 2.Hedén P, Jernbeck J, Hober M. Breast augmentation with anatomical cohesive gel implants: the world’s largest current experience. Clin Plast Surg. 2001;28:531–552. [PubMed] [Google Scholar]
- 3.Baek WY, Byun IH, Seok Kim Y, et al. Variance of the pectoralis major in relation to the inframammary fold and the pectoralis minor and its application to breast surgery. Clin Anat. 2017;30:357–361. [DOI] [PubMed] [Google Scholar]
- 4.Maclin MM, II, Deigni OA, Bengtson BP. The laminated nature of the pectoralis major muscle and the redefinition of the inframammary fold: clinical implications in aesthetic and reconstructive breast surgery. Clin Plast Surg. 2015;42:465–479. [DOI] [PubMed] [Google Scholar]
- 5.Hakelius L, Ohlsén L. Tendency to capsular contracture around smooth and textured gel-filled silicone mammary implants: a five-year follow-up. Plast Reconstr Surg. 1997;100:1566–1569. [DOI] [PubMed] [Google Scholar]
- 6.Hedén P. Commentary on: functional and volumetric analysis of the pectoralis major muscle after submuscular breast augmentation. Aesthet Surg J. 2017;37:662–664. [DOI] [PubMed] [Google Scholar]
- 7.Davidson BA. Subpectoral breast augmentation emphasizing muscle preservation. Aesthet Surg J. 1997;17:264–266. [DOI] [PubMed] [Google Scholar]
- 8.Hojo T, Nakashima T, Tsuruno T. A statistical study on anatomical variation in the origin of the Japanese pectoralis minor muscle. J UOEH. 1987;9:315–319. [DOI] [PubMed] [Google Scholar]
- 9.Borstad JD. Measurement of pectoralis minor muscle length: validation and clinical application. J Orthop Sports Phys Ther. 2008;38:169–174. [DOI] [PubMed] [Google Scholar]
- 10.Nanigian BR, Wong GB, Khatri VP. Inframammary crease: positional relationship to the pectoralis major muscle origin. Aesthet Surg J. 2007;27:509–512. [DOI] [PubMed] [Google Scholar]
- 11.Mallucci P, Branford OA. Concepts in aesthetic breast dimensions: analysis of the ideal breast. J Plast Reconstr Aesthet Surg. 2012;65:8–16. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez ER, Howland N, Kaltwasser K, et al. Anatomy of the sternal origin of the pectoralis major: implications for subpectoral augmentation. Aesthet Surg J. 2014;34:1179–1184. [DOI] [PubMed] [Google Scholar]
- 13.Sim HB, Hwang K, Huan F, et al. Anatomy and tensile strength of the abdominal head of the pectoralis major muscle in relation to transaxillary breast augmentation. Aesthetic Plast Surg. 2013;37:359–363. [DOI] [PubMed] [Google Scholar]
- 14.Lindsey JT. The case against medial pectoral releases: a retrospective review of 315 primary breast augmentation patients. Ann Plast Surg. 2004;52:253–256. [DOI] [PubMed] [Google Scholar]
- 15.Moliver CL, Sanchez ER, Kaltwasser K, et al. A muscular etiology for medial implant malposition following subpectoral augmentation. Aesthet Surg J. 2015;35:NP203Np203–NP203NP210. [DOI] [PubMed] [Google Scholar]
- 16.Montemurro P, Avvedimento S, Hedén P, et al. A four-layer wound closure technique with barbed sutures for stable reset of the inframammary fold in breast augmentation. Aesthet Surg J. 2016;36:966–971. [DOI] [PubMed] [Google Scholar]
- 17.Madsen RJ, Jr, Chim J, Ang B, et al. Variance in the origin of the pectoralis major muscle: implications for implant-based breast reconstruction. Ann Plast Surg. 2015;74:111–113. [DOI] [PubMed] [Google Scholar]
- 18.Spear SL, Sher SR, Al-Attar A. Focus on technique: supporting the soft-tissue envelope in breast reconstruction. Plast Reconstr Surg. 2012;130:89S89s–89S94S. [DOI] [PubMed] [Google Scholar]
- 19.Price CI, Eaves FF, III, Nahai F, et al. Endoscopic transaxillary subpectoral breast augmentation. Plast Reconstr Surg. 1994;94:612–619. [DOI] [PubMed] [Google Scholar]