Figure 1.
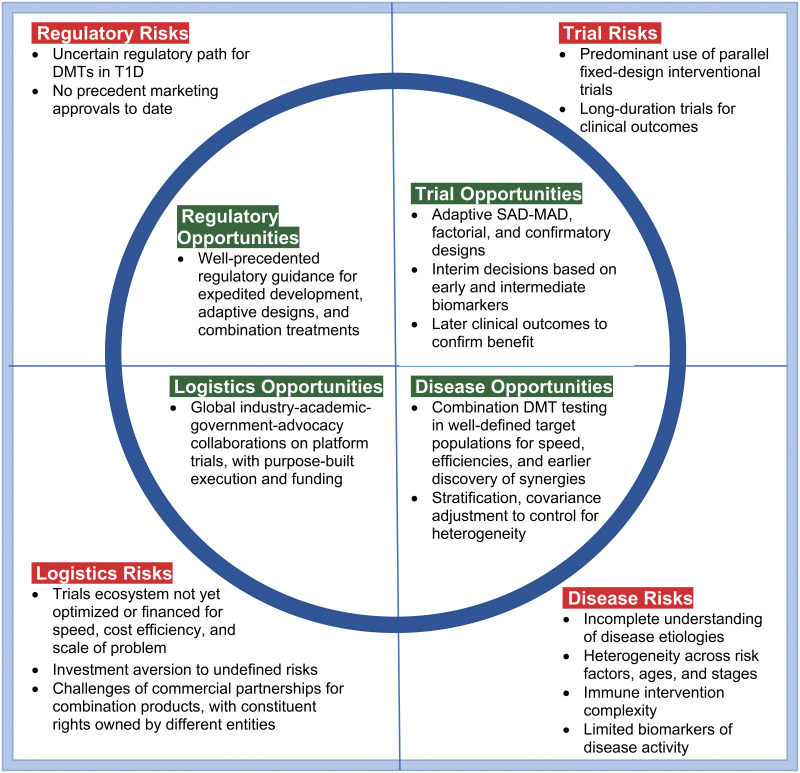
Daunting “square peg” challenges and “rounding” opportunities for the clinical trials ecosystem for DMTs in T1D are best addressed collectively in trials design. For disease risks, broad expert consensus exists that combination DMTs will be needed for durable, more clinically meaningful effects. Baseline biomarkers are available as stratification factors to ameliorate heterogeneity issues. The potential exists for extensive, systematic biomarker data mining and validation across trials to rapidly inform progress and define decision criteria for subsequent trials. Available biomarkers have shown correlation with important clinical outcomes, and further biomarker research/validation can be incorporated into clinical program plans and platform protocols for careful dose selection, early proof of concept, and confirmatory clinical efficacy. C-peptide AUC is a widely accepted clinical end point and can be used at 6-month intervals. Factors to manage trial risks are broader use of adaptive designs, early dose-finding designs, factorial designs, and platform protocols for purpose-built speed and efficiency. In terms of logistical risks, platform protocols are working well in other indications (e.g., pancreatic cancer), with emerging efforts in T1D via INNODIA, T1DUK, and Global Platform for the Prevention of Autoimmune Diabetes. Global, adaptive platform protocols are needed for early (phase 1/2a) and late (phase 2b/3) product development. Concerning regulatory risks, T1D qualifies as a serious disease, availing expedited regulatory support. Adaptive designs have been well accepted in other indications, and there are multiple facilitative regulatory guidelines for treatment combinations.