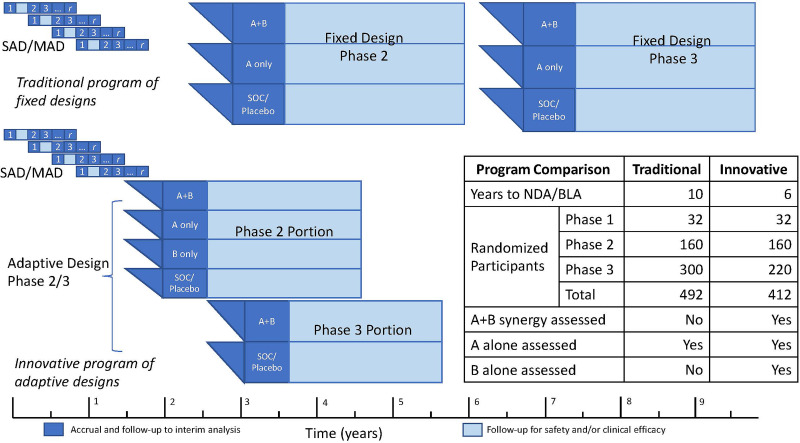
Figure 2.
Conceptual comparison of traditional and innovative program plans for a combination DMT in new-onset T1D to achieve a New Drug Application (NDA) or Biologics Licensing Application (BLA). Both programs provide randomized, treatment-masked phase 2 and phase 3 designs, with inferences powered for 0.5 SD effect sizes for main effect tests (phase 2: 85% power; phase 3: 90% power). The innovative program plan makes full use of the adaptive designs and logistics ideas described here. The phase 2 factorial design provides inferences for A and B main effects as well as an estimate of synergy sufficient to support a conditional power calculation for a phase 3 A+B versus SOC/placebo inference. First, a SAD–MAD combination trial with constituent 1 and constituent 2 would initiate the clinical program (program month 1). Positive interim results from an interim analysis of the SAD–MAD cohorts (3-month safety and robust early biomarker effects) could support application for breakthrough designation (program month 9). Second, a phase 2/3 adaptive trial of constituent 1 and constituent 2, where the phase 2 portion is a 2 × 2 factorial design, including the combination group, the two monotherapy groups, and a placebo group, initiated based on 6-month follow-up data from the last SAD–MAD dose–escalation cohort (no later than program month 15). The phase 3 portion would be a two-group comparison of the combination versus placebo, initiated based on a phase 2 interim safety and efficacy analysis (of accrued safety data and 6-month stimulated C-peptide AUC) at program month 30. Both the SAD–MAD study and the phase 2/3 study would be planned for up to 3 years of follow-up. However, the decision point for starting the phase 3 portion would be positive phase 2 interim safety and efficacy analysis at 15 months from phase 2 first patient first visit (program month 30). This information, along with longer-term SAD–MAD follow-up data, may then support an application for accelerated approval. The marketing application would be based on a phase 3 interim analysis planned at the 1-year follow-up. With the results supporting expedited review for accelerated approval, the marketing application could be as early as program month 45. Final approval (following an earlier conditional approval) might be based on the 3-year follow-up results from the phase 2/3 design (at program month 70). The traditional program is exemplified by typical “white space” between studies and parallel phase 2 and 3 designs where synergy is not estimable.