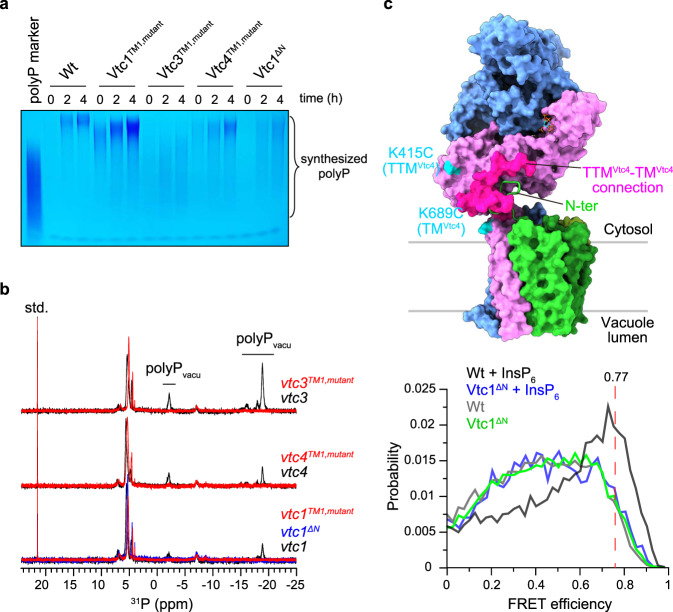
Fig. 5. Mechanistic coupling of polyP synthesis and membrane translocation.
a Assay of VTC-catalyzed polyP synthesis. 5 μM protein complex were used and reactions were performed with required times in the presence of 1 mM InsP6. Wt, Vtc1/Vtc3/Vtc4; Vtc1TM1,mutant, Vtc1K24A/R31A/Vtc3/Vtc4; Vtc3TM1,mutant, Vtc1/Vtc3K694A/K698A/R705A/R709A/Vtc4; Vtc4TM1,mutant, Vtc1/Vtc3/Vtc4K622A/R629A; Vtc1ΔN, Vtc1Δ1–21/Vtc3 /Vtc4. Source data are provided as a Source Data file. The experiments were repeated three times independently with similar results. b In-cell 31P-NMR measurement of vacuolar polyP in yeast strains. vtc3 and vtc3TM1,mutant, vtc4 and vtc4TM1,mutant, vtc1, vtc1TM1,mutant and Vtc1ΔN were complemented into the vtc2Δvtc3Δ, vtc2Δvtc4Δ, vtc2Δvtc1Δ background strains, respectively. Chemical shifts of vacuolar polyP are denoted. MDP was used as the std. reference. c smFRET analysis of VTC conformational changes. Top: Sites for fluorophores conjugation are highlighted in cyan on the molecular surface representation of InsP6-activated VTC complex. The connection between TTMVtc4 and TMVtc4 (residues 472–618) is represented in magenta surface. The N-terminus of Vtc1A protomer (N-ter, residues 1–21) is shown in cartoon representation. Bottom: smFRET profiles of VTC complex (Wt) in the presence and absence of 1 mM InsP6 are colored in black and gray lines, respectively. smFRET profiles of VTC complex carrying Vtc1 N-terminus deletion (Vtc1ΔN) in the presence and absence of 1 mM InsP6 are colored in blue and green lines, respectively. Red dashed line indicates the position of FRET efficiency of 0.77.