FIGURE 4.
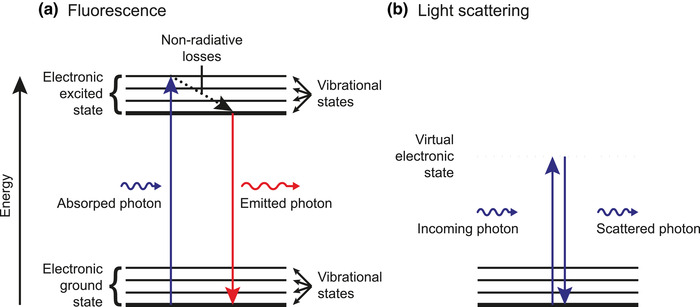
Jablonski diagrams of fluorescence and light scattering by a molecule. (A) The horizontal lines represent the levels of energy states of a molecule. The lowest energy level corresponds to the ground state (thick line). When the electrons are in the lowest possible orbital, the molecule is in the electronic ground state. Within the electronic ground state, the molecule can occupy different vibrational states with different energies, representing different types of periodic motions of the molecule. In the process of fluorescence, an incoming photon is absorbed and temporarily excites both the electronic and vibrational state of a molecule. Next, the molecule loses energy and therefore decays to the lowest vibrational state without radiative emission (dotted arrow). Finally, the molecule relaxes to the ground state under the emission of a photon. Due to the non‐radiative energy loss, the emitted photon has a lower energy and longer wavelength than the incoming photon. (B) In the process of light scattering, an incoming photon excites the molecule to a virtual electronic state (dashed line), which is instantaneously followed by relaxation of the molecule to the ground state and emission of a photon. The energy and wavelength of the incoming and scattered photon are the same.
