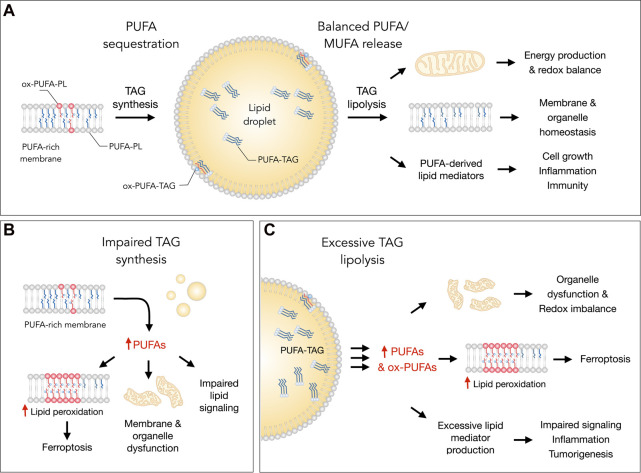
FIGURE 1.
Lipid droplets (LDs) control essential cellular processes and protect from ferroptosis by balancing polyunsaturated fatty acid (PUFA) sequestration and release. (A) Under homeostatic conditions, the biosynthesis of triacylglycerols (TAGs) enriched with PUFAs (PUFA-TAGs) and their packaging in LDs may lower the abundance of oxidizable PUFAs esterified in membrane phospholipids (PUFA-PLs). In principle, already oxidized PUFAs esterified in phospholipids (ox-PUFA-PLs) could also be transferred into LDs and stored as oxidized TAGs (ox-PUFA-TAGs). On the other hand, the balanced release of monounsaturated fatty acids (MUFAs) and PUFAs via TAG lipolysis maintains proper membrane composition, thereby preventing lipid peroxidation and reducing ferroptosis sensitivity. PUFA release from LDs also supports energy production, mitochondrial function and the redox balance. In addition, lipolysis also delivers PUFAs into oxygenation pathways that convert these FAs into signaling mediators, such as prostaglandins and leukotrienes, which regulate various cellular functions, including cell growth, inflammation and immunity. (B) Under various stress conditions, impaired TAG synthesis may lead to an enrichment of membranes with PUFAs that can result in elevated lipid peroxidation and ferroptosis. Excess PUFAs may also overload mitochondria and other organelles, as well as disrupt the biosynthesis of lipid mediators. (C) Excessive breakdown of LDs by neutral lipases (at the lipid droplet surface) or acid lipases (in the lysosome) during acute or chronic stress may disrupt organelle function, impair redox signaling and metabolism, and enrich membranes with PUFAs. This can increase lipid peroxidation levels and sensitivity to ferroptosis. The release of excess PUFAs from LDs may also stimulate the production of pro-inflammatory and pro-tumorigenic lipid mediators, which can overstimulate mitogenic, inflammatory and other signaling pathways.