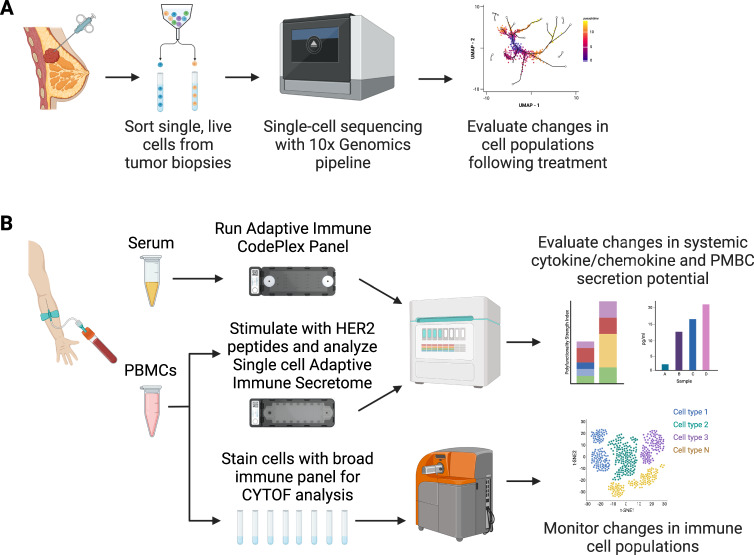
Fig. 1. Schematic for immune analysis of biopsies and blood samples from safety lead-in cohort form pembrolizumab plus VRP-HER2 trial.
A Biopsies pre- and post-vaccination were digested and single live cells were sorted and were then subjected to single-cell RNA sequencing. B Serum was analyzed for alterations of systemic cytokines. PBMCs were stimulated by an overlapping panel of HER2 peptides and functional cytokine/marker alterations of single cells were assessed by single cell secretome ELISA and Cytometry Time of Flight (CYTOF).