Figure 1.
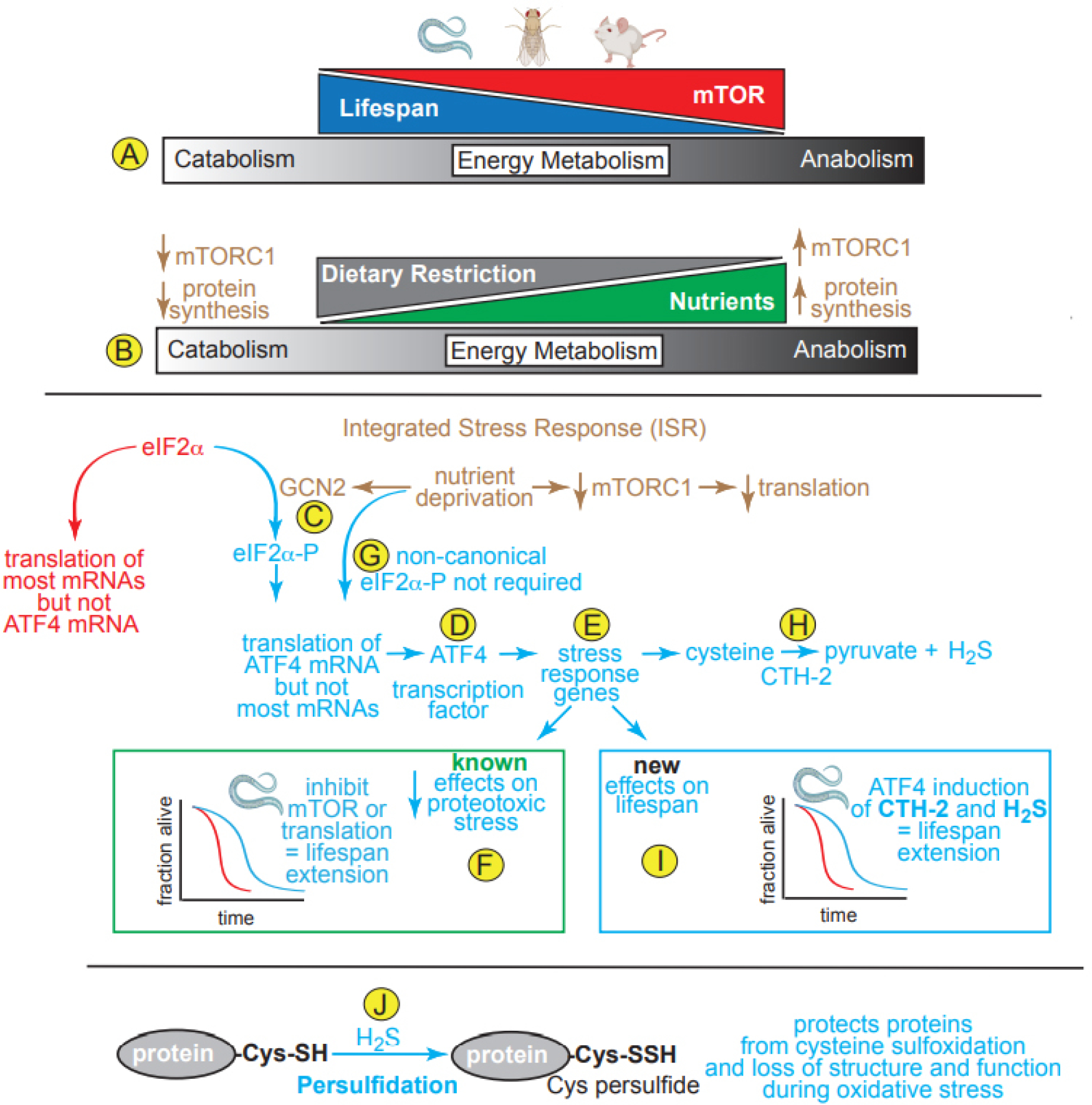
(A, B) Effects on Lifespan of mTOR, protein synthesis and dietary restriction on catabolic and anabolic energy metabolism. (C) The integrated stress response (ISR) involves activation of GCN2, a kinase that is activated upon nutrient deprivation, phosphorylates eIF2a, which inhibits most mRNA translation, but allows translation of a few mRNAs, such as that encoding ATF4. (D) ATF4 is a transcription factor that (E) induces numerous stress-response genes required for cells to adjust to decreased nutrient availability. (F) Inhibition of mTOR, which then decreases translation, or inhibition of translation direction with protein synthesis inhibitors, such as cycloheximide, decreases proteotoxic stress in C. elegans, leading to lifespan extension. (G) Statzer et al.[4] showed a new, non-canonical mechanism of increasing ATF4 translation that does not require eIF2a phosphorylation. (H) One of the genes Statzer et al.[4] found to be induced by ATF4 in C. elegans is CTH-2, which catalyzes the formation of H2S. (I) Among the new findings reported by Statzer et al.[4] are that ATF4 induced by inhibiting mTOR or translation induces CTH-2, which increases H2S and lifespan extension; (J) H2S contributes to persulfication of cysteine thiols on proteins, which affects their structures and functions in protective ways.
