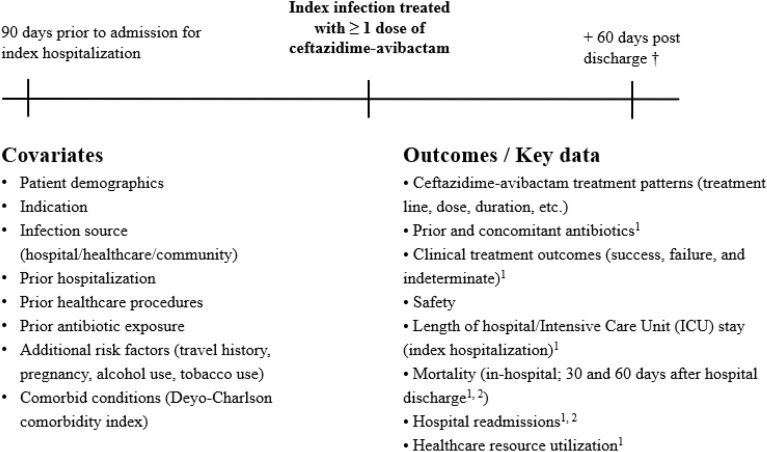
Fig. 1.
Study design. 1Indicates outcome that was examined for patients exposed to ceftazidime–avibactam for ≥ 72 h. 2Information about hospital readmissions or death after hospital discharge not available via medical chart abstraction was ascertained by contacting the patient or their legal representative by phone > 60 days after hospital discharge. † Patients followed from ceftazidime–avibactam initiation until 60 days after hospital discharge or another censoring event (in-hospital death, withdrawal from the study, or loss-to-follow-up, whichever occurs first). Data had been abstracted from patient medical charts. Note: Data collection began 01 Jan 2018 or since the date of the launch if after 01 Jan 2018