Abstract
The hemostatic effect of palliative radiation therapy (RT) for unresectable gastric cancer is unclear. We performed palliative RT (20 Gy in 5 fractions or 30 Gy in 10 fractions) in 7 consecutive patients with bleeding. The number of blood transfusions decreased significantly post‐RT, supporting the hemostatic effect of palliative RT.
Keywords: gastric cancer, hemostasis, palliation, radiation therapy
We reported seven patients with gastric cancer with bleeding treated with palliative radiation therapy. The number of blood transfusions decreased significantly post‐radiation therapy, supporting the hemostatic effect of palliative radiation therapy.
1. INTRODUCTION
Advanced gastric cancer induces various symptoms, such as bleeding, anorexia, obstruction, and pain. In particular, bleeding from gastric cancer not only compromises the quality of life but also results in a life‐threatening condition. 1
Palliative treatment options for bleeding from advanced gastric cancer include surgery, endoscopic hemostasis, transcatheter embolotherapy, and radiation therapy (RT). 1 Surgery is an effective treatment for bleeding from gastric cancer; however, it is eligible only for patients with a good performance status (PS). 2 Conversely, endoscopic hemostasis, transcatheter embolotherapy, and RT can be administered even to inoperable patients.
The rate of successful hemostasis by endoscopic treatment and by embolotherapy is reported to be 31%–100% 3 , 4 , 5 and 40%–100%, 1 respectively. Hemostatic RT is not used as commonly as those treatments. 6 There have been several studies reporting the efficacy of RT on hemostasis as being approximately 50%–80%. 1 However, the dose and fractionation have varied widely among studies, ranging from 8 Gy in 1 fraction to 60 Gy in 30 fractions, making the efficacy of this treatment obscure. In the clinical setting, the most commonly used dose fractionation includes 20 Gy in 5 fractions and 30 Gy in 10 fractions.
We retrospectively analyzed the efficacy of palliative RT on hemostasis in seven patients with unresectable gastric cancer treated using dose fractionations.
2. CASE SERIES
Patients with pathologically‐confirmed unresectable gastric cancer who met the following criteria were retrospectively analyzed: (i) bleeding from gastric cancer confirmed by endoscopic or bleeding signs as hematemesis or melena; (ii) treated with palliative RT for hemostatic purposes at Sano Kosei General Hospital between April 2019 and December 2021; and (iii) followed for at least 2 months post‐RT. As a result, 7 patients were considered eligible for the analysis, and the median follow‐up period was 5 (range, 2–9) months (Table 1).
TABLE 1.
Patient's characteristics (n = 7).
| Gender (male/female) | 7/0 |
| Melena | |
| +/− | 4/3 |
| Irradiation dose | |
| 20 Gy/5 Fr | 3 |
| 30 Gy/10 Fr | 4 |
| Age, median (range) | 80 (71–88) |
| 20 Gy/5 Fr | 81 (78–82) |
| 30 Gy/10 Fr | 79 (71–88) |
| Performance status | |
| 20 Gy/5 Fr | |
| 0 | 1 |
| 1 | 1 |
| 2 | 1 |
| 30 Gy/10 Fr | |
| 0 | 2 |
| 1 | 2 |
| 2 | 0 |
| Follow‐up period, median (range) | 5 months (2–9 months) |
| 20 Gy/5 Fr | 3 months (2–4 months) |
| 30 Gy/10 Fr | 6 months (2–9 months) |
Abbreviation: Fr, fraction.
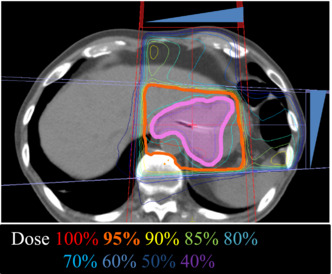
All patients were treated with external‐beam three‐dimensional conformal RT using 10 MV‐photons. Treatment planning was performed using Monaco (Elekta Co., Ltd.). The clinical target volume was defined as the gross tumor volume with 5‐mm outward margins identified by computed tomography (Figure 1). Using a Synergy irradiator (Elekta), 3 and 4 patients received a total of 20 Gy in 5 fractions and 30 Gy in 10 fractions, respectively, in a 5‐fractions‐per‐week schedule. Irradiation was performed on an empty stomach in all cases and delivered to box four fields or parallel opposing fields. Whether to use 20 or 30 Gy was decided based on the condition of each patient. No patients received re‐irradiation for gastric cancer.
FIGURE 1.
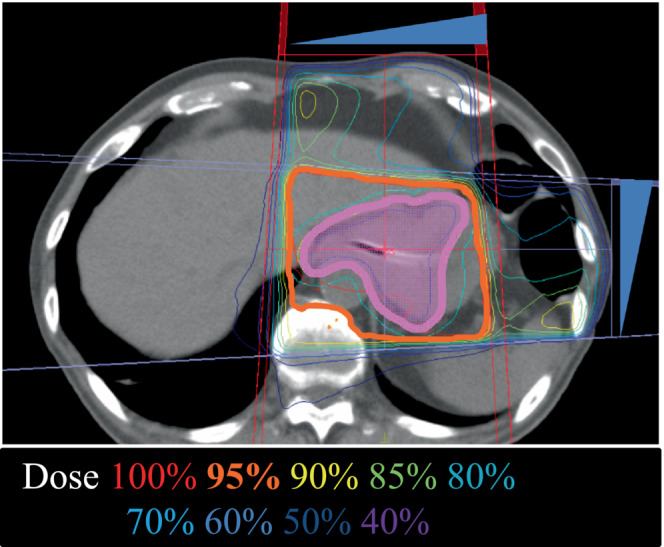
A representative image of the radiation therapy (RT) plan (Case #4). Solid magenta indicates the clinical target volume. Blue triangles show the wedge for beam intensity adjustment (45° for each).
Four patients had melena before radiation therapy, and the amount of melena had reduced after treatment. In all patients, the number of transfusions was reduced or remained transfusion‐free, which improved the patients' quality of life. Compared to 2 months before RT, the number of blood transfusions significantly decreased in the 2 months after RT (2.42 ± 1.72 vs. 0.14 ± 0.38, p = 0.0121 assessed by the paired t‐test; Table 2). In three‐quarters of the 30‐Gy patients, hemoglobin levels increased after irradiation. In contrast, the hemoglobin levels in the 20‐Gy patients continuously increased in only one case (Figure 2). No adverse events as assessed by the Common Terminology Criteria for Adverse Event (version 4.0) were observed in any patients.
TABLE 2.
The number of blood transfusions.
| Before RT (2 months) | Post‐RT (2 months) | |
|---|---|---|
| 30 Gy/10 Fr | ||
| Case #1 | 3 | 1 |
| Case #2 | 4 | 0 |
| Case #3 | 0 | 0 |
| Case #4 | 4 | 0 |
| 20 Gy/5 Fr | ||
| Case #5 | 0 | 0 |
| Case #6 | 3 | 0 |
| Case #7 | 3 | 0 |
| Average ± SD | 2.42 ± 1.72 | 0.14 ± 0.38 |
Abbreviation: Fr, fraction; RT, radiation therapy.
FIGURE 2.
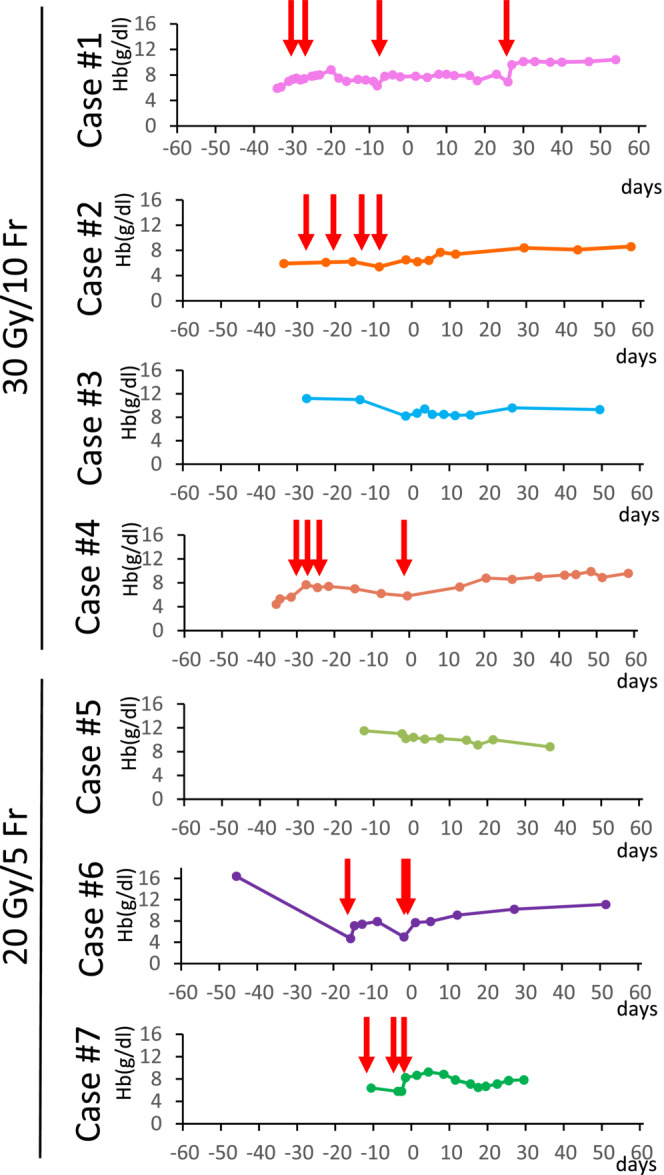
Kinetics of hemoglobin levels within 2 months pre‐ and post‐radiation therapy (RT). Day 1 indicates the day of initiation of RT. Red arrows indicate blood transfusion.
3. DISCUSSION
Our data indicate that RT of 20 Gy in 5 fractions and 30 Gy in 10 fractions can prevent a rapid decline in the hemoglobin level and reduce the frequency of blood transfusion. From this perspective, palliative RT can contribute to improving the quality of life of patients with advanced gastric cancer with bleeding.
There have been several reports on RT for advanced gastric cancer with bleeding. Lee et al. analyzed hemoglobin and blood transfusion frequency after RT for 57 patients submitted to palliative RT for gastric bleeding. 7 The authors reported that the mean hemoglobin levels before, immediately after, and one and 2 months after RT were significantly higher than before RT. No significant differences in re‐bleeding rates were observed according to the total dose (17.5–45 Gy), fractional dose (1.8–5 Gy), or fraction number (4–25 fractions). Tey et al. investigated approximately 50 patients with advanced gastric cancer. 8 In that study, the median survival duration was 85 days, and 80% of patients with bleeding responded to RT. Two patients (5%) had grade 3 anorexia and gastritis, but their symptoms resolved after a week. They received 36 Gy in 12 fractions, and the authors concluded that RT was effective and well‐tolerated. In Japan, no guideline has yet been established for hemostatic RT for advanced gastric cancer. Taken together, these data indicate that RT at a total of 20–30 Gy is effective in alleviating bleeding from advanced gastric cancer.
The optimal dose for achieving hemostasis in cases of advanced gastric cancer is controversial, as a review by Tey et al. reported wide inter‐study variations in dose fractionation. 9 Fraction sizes ranged from 1.8 to 8 Gy, and total doses ranged from 8 Gy to 50 Gy, with the most common dose fraction regimen being 30 Gy in 10 fractions. The response to RT for bleeding ranged from 50% to 80.6%. The median duration of response ranged from 1.5 to 11.4 months. This review concluded that there was no marked difference in the response rate of bleeding between regimens with a high biological equivalent dose (BED) of ≥39 Gy versus those with a low BED of <39 Gy. Furthermore, low‐BED regimens appear to be adequate for symptom palliation. The BED for 20 Gy in 5 fractions and 30 Gy in 10 fractions, as was administered to the patients in our study, was 28 and 39 Gy, respectively. Although 30 Gy in 10 fractions is classified as a high BED of ≥39 Gy, no Grade ≥3 complications were observed in our study.
In summary, we report consecutive seven patients with gastric cancer with bleeding treated with palliative RT (20 Gy in 5 fractions or 30 Gy in 10 fractions). The number of blood transfusions decreased significantly post‐RT, supporting the hemostatic effect of palliative RT.
AUTHOR CONTRIBUTIONS
Takahiro Oike: Data curation; methodology; project administration; supervision. Yuta Hisatake: Conceptualization; investigation; resources. Toshihiko Higashizawa: Conceptualization; methodology; resources. Ken Teramoto: Conceptualization; methodology; resources. Yukishige Okamura: Conceptualization; methodology; resources. Tatsuya Ohno: Conceptualization; formal analysis; investigation; methodology; project administration; resources; supervision.
FUNDING STATEMENT
None declared.
CONFLICT OF INTEREST STATEMENT
None declared.
INFORMED CONSENT
This study was approved by the Ethics Review Committee of Sano Kosei General Hospital (approval number: 202109). Written informed consent is declared. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
CONSENT STATEMENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGMENTS
We thank Mr. Nobuyuki Sawai, Mr. Michiyuki Wada, Mr. Naonori Haraguchi, Ms. Takami Sugimoto, Ms. Yuki Suzuki, Ms. Erina Ishikawa, and Ms. Mariko Kaneko of Sano Kousei General Hospital for their clinical assistance. This work was supported by Gunma University Heavy Ion Medical Center.
Ota N, Adachi A, Oike T, et al. Palliative radiation therapy for bleeding from gastric cancer: A case series of seven patients. Clin Case Rep. 2023;11:e6955. doi: 10.1002/ccr3.6955
DATA AVAILABILITY STATEMENT
Raw data are not available, as restricted by the study protocol approved by the Ethics Review Committee of Sano Kosei General Hospital (approval number: 202109).
REFERENCES
- 1. Kawabata H, Hitomi M, Motoi S. Management of bleeding from unresectable gastric cancer. Biomedicine. 2009;7:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thumurthy SG, Chaudry MA, Chau I, et al. Does surgery have a role in managing incurable gastric cancer? Nat Rev Clin Oncol. 2015;12:676‐682. [DOI] [PubMed] [Google Scholar]
- 3. Koh KH, Kim K, Kwon DH, et al. The successful endoscopic hemostasis factors in bleeding from advanced gastric cancer. Gastric Cancer. 2013;28:1489‐1495. [DOI] [PubMed] [Google Scholar]
- 4. Song IJ, Kim HJ, Cho SJ, et al. Clinical outcomes of endoscopic hemostasis for bleeding in patients with unresectable advanced gastric cancer. J Gastric Cancer. 2017;17:374‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim YJ, Park JC, Kim EH, Shin SK, Lee SK, Lee YC. Hemostatic powder application for control of acute upper gastrointestinal bleeding in patients with gastric malignancy. Endosc Int Open. 2018;6:E700‐E705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kosugi T, Shikama N, Saito T, et al. Nationwide surgery in Japan of palliative radiotherapy for bleeding in gastrointestinal and genitourinary tumor patients. World J Oncol. 2016;7:29‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee J, Byun HK, Koom WS, Lee YC, Seong J. Efficacy of radiotherapy for gastric bleeding associated with advanced gastric cancer. Radiat Oncol. 2021;16:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tey J, Zheng H, Soon YY, et al. Palliative radiotherapy in symptomatic locally advanced gastric cancer: a phase II trial. Cancer Med. 2019;8:1447‐1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tey J, Soon YY, Koh WY, et al. Palliative radiotherapy for gastric cancer: a systematic review and meta‐analysis. Oncotarget. 2017;8:25797‐25805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data are not available, as restricted by the study protocol approved by the Ethics Review Committee of Sano Kosei General Hospital (approval number: 202109).


