Abstract
Purpose:
This exploratory study aims to investigate variations in voice production in the presence of background noise (Lombard effect) in individuals with nonphonotraumatic vocal hyperfunction (NPVH) and individuals with typical voices using acoustic, aerodynamic, and vocal fold vibratory measures of phonatory function.
Method:
Nineteen participants with NPVH and 19 participants with typical voices produced simple vocal tasks in three sequential background conditions: baseline (in quiet), Lombard (in noise), and recovery (5 min after removing the noise). The Lombard condition consisted of speech-shaped noise at 80 dB SPL through audiometric headphones. Acoustic measures from a microphone, glottal aerodynamic parameters estimated from the oral airflow measured with a circumferentially vented pneumotachograph mask, and vocal fold vibratory parameters from high-speed videoendoscopy were analyzed.
Results:
During the Lombard condition, both groups exhibited a decrease in open quotient and increases in sound pressure level, peak-to-peak glottal airflow, maximum flow declination rate, and subglottal pressure. During the recovery condition, the acoustic and aerodynamic measures of individuals with typical voices returned to those of the baseline condition; however, recovery measures for individuals with NPVH did not return to baseline values.
Conclusions:
As expected, individuals with NPVH and participants with typical voices exhibited a Lombard effect in the presence of elevated background noise levels. During the recovery condition, individuals with NPVH did not return to their baseline state, pointing to a persistence of the Lombard effect after noise removal. This behavior could be related to disruptions in laryngeal motor control and may play a role in the etiology of NPVH.
Supplemental Material:
Vocal hyperfunction refers to voice production characterized by excessive perilaryngeal musculoskeletal activity (Hillman et al., 1989, 2020; Oates & Winkworth, 2008). A recently updated theoretical framework for vocal hyperfunction specifies the existence of two subtypes based on etiological and pathophysiological factors (Hillman et al., 2020). The first subtype is defined as phonotraumatic vocal hyperfunction (PVH) and is associated with the formation of benign vocal fold lesions through chronic tissue trauma. The second subtype is nonphonotraumatic vocal hyperfunction (NPVH), which is associated with hyperactivity or imbalanced activity of the perilaryngeal muscles in the absence of vocal fold tissue trauma or other overt structural or neurological issues that could affect phonation (Hillman et al., 2020). NPVH is also referred to as primary muscle tension dysphonia (Hillman et al., 2020; Morrison et al., 1983) and has been associated with different precipitating factors, including inefficient phonatory function (Espinoza et al., 2017; Hillman et al., 2020), psychological stress-related factors (Demmink-Geertman & Dejonckere, 2002), impaired auditory discrimination (Abur et al., 2021), and sensorimotor deficits (Abur et al., 2021; McKenna et al., 2020; Stepp et al., 2017; Tam et al., 2018; Ziethe et al., 2019).
Auditory–Motor Control as a Factor in Vocal Hyperfunction
Recent studies suggest that the imbalanced laryngeal muscle activity in vocal hyperfunction could be related to impaired auditory discrimination and disrupted auditory–motor integration mechanisms, which potentially result in altered vocal production (Abur et al., 2021; Stepp et al., 2017). This idea is based on the variability of the vocal responses to altered auditory feedback using a sustained pitch-shift paradigm (Stepp et al., 2017). Moreover, recent studies reported that some individuals with vocal hyperfunction exhibit worse auditory discrimination and atypical adaptive responses to pitch-shift paradigms in comparison to participants with typical voice (Abur et al., 2021). In addition, individuals with NPVH show greater cortical activity in prefrontal, temporal, and limbic areas than vocally typical controls during phonatory tasks (Kryshtopava et al., 2017; Roy et al., 2019; Samargia et al., 2016), further implicating atypical speech motor control mechanisms. Furthermore, individuals with vocal hyperfunction (both PVH and NPVH subtypes) tend to exhibit reduced auditory discrimination abilities (Abur et al., 2021; Tam et al., 2018) and generate more variability in phonemic voicing targets than individuals with typical voices (McKenna et al., 2020). These results suggest that individuals with vocal hyperfunction might have reduced acuity to auditory feedback differences and larger auditory targets in the context of laryngeal motor control. These prior efforts have explored pitch motor control using an altered auditory feedback paradigm. However, the impact of auditory feedback on control of vocal intensity is less explored for voice disorders. Assessing vocal function when the sound pressure level (SPL) target is involuntarily altered by the background noise in individuals with vocal hyperfunction provides a different avenue to investigate laryngeal motor control. Although pitch perturbation experiments suggest that both PVH and NPVH groups can exhibit auditory–motor integration problems, we decided to study these patient groups separately for vocal intensity given the different physical manifestations they exhibit (Espinoza et al., 2017). Given the complex etiology of NPVH with a psychological predisposition of introversion, constrain, and anxiety (Roy & Bless, 2000), we hypothesize that these patients can be more sensitive and produce more stress-reactive responses to changes in environmental conditions (e.g., background noise) and are thus the focus of this first study. Future efforts will be devoted to studying PVH.
The involuntary increase of vocal intensity due to masking noise is known as the Lombard effect (Lombard, 1911). This phenomenon has been considered as an adaptive communicative mechanism, which depends on the energy and spectral composition of the sound masker (Meekings et al., 2016; Stowe & Golob, 2013), the linguistic context of the message (Patel & Schell, 2008), and the type/intention of the communicative interaction between the speaker and the audience (Garnier et al., 2010). In addition to variations in the intensity of voice, speech changes related to the Lombard effect also include increases in fundamental frequency (Stowe & Golob, 2013), decreases in spectral tilt (Lu & Cooke, 2008), and increases in the length of syllables and vowels (Garnier & Henrich, 2014). The rate of change of vocal SPL for a decibel change in environmental noise level (Lombard slope) depends on the type of environmental noise to which individuals are exposed (Sato & Bradley, 2008). In a recent ambulatory voice study of individuals with typical vocal function in a naturalistic environment (Whittico et al., 2020), the Lombard slope was 0.54 dB/dB, which agrees with laboratory studies and expected compensatory mechanisms due to self-perception of voice (Lindstrom et al., 2011). Of note, relatively large interindividual differences in the Lombard slope have been observed in occupational environments, that is, preschool teachers in classrooms (Lindstrom et al., 2011). Although the Lombard slope was 1.0 dB/dB for some teachers, others exhibited different slopes. In certain cases, the voice SPL of these individuals remained relatively high even when the acoustic noise level had decreased. In other words, the Lombard effect persisted even when the presumed trigger of the adaptive behavior was no longer present.
A typical persistence of adaptive vocal responses to auditory feedback alterations (such as changes in fundamental frequency) has been previously noted (Behroozmand & Sangtian, 2018; Jones & Keough, 2008; Nasir et al., 2013). Based on neurocomputational models of speech motor control (Houde & Nagarajan, 2011; Parrell et al., 2019; Tourville & Guenther, 2011), this phenomenon suggests that the after-effect of vocal adaptive responses may reflect sensorimotor reprogramming of feedforward motor commands in response to auditory feedback alterations (Behroozmand & Sangtian, 2018). Different explanations can be provided for the persistence of Lombard effect, and the possible physiological mechanisms underpinning the mismatch between vocal level and noise level have not been postulated. Considering that imbalanced laryngeal muscle activity in NPVH might be a consequence of disrupted auditory–motor integration mechanisms, we hypothesize that the persistence of the Lombard effect in the absence of noise is a potential characteristic of some individuals with NPVH.
Aerodynamic and High-Speed Videoendoscopy Measures of Vocal Function
Aerodynamic measures of vocal function have proven to be useful in differentiating individuals with NPVH from those with typical voices. These aerodynamic measures are estimated from the glottal airflow signal, including (a) unsteady peak-to-peak airflow amplitude (ACFL), which is related to the amplitude of the vibratory motion of the vocal folds; (b) flow-based open quotient (OQf), the ratio of the open phase to the total duration of a vibratory cycle in the glottal airflow signal; (c) maximum flow declination rate (MFDR), the fastest rate of change of the airflow during the closing phase of the vibratory cycle (Holmberg et al., 1988; Sapienza & Stathopoulos, 1994), which is related to vocal fold tissue velocity and collision pressure (Galindo et al., 2017; Zañartu et al., 2014); and (d) subglottal pressure (SGP), the driving pressure to sustain vocal fold vibration, which is related to vocal fold collision pressure (Galindo et al., 2017; Mehta et al., 2019; Motie-Shirazi et al., 2019).
In acoustic environments with low levels of background noise, ACFL, MFDR, OQf, and SGP are correlated with voice SPL (Espinoza et al., 2017; Hillman et al., 1989; Rosenthal et al., 2014; Sapienza & Stathopoulos, 1994; Sundberg et al., 2005), and they can differentiate between individuals with typical voices and individuals with NPVH (Espinoza et al., 2017). Furthermore, Espinoza et al. (2017) used SPL-normalized aerodynamic measures in addition to the standard parameters described above based on earlier work by Hillman et al. (1989). These SPL-normalized measures are ratios between the voice SPL output and each measure that are designed to control for overall SPL while capturing the efficiency in converting aerodynamic energy into acoustic SPL. These measures were successfully used for differentiating typical voices against two groups (PVH and NPVH) of patients with vocal hyperfunction. Examining the impact of the Lombard effect on the measures that differentiate the two types of vocal hyperfunction may provide new insights into their respective underlying mechanisms.
Laryngeal high-speed videoendoscopy (HSV) has been used in studies to differentiate typical voices from disordered voices (Bonilha et al., 2012; Švec et al., 2007; Yamauchi et al., 2016; Zacharias et al., 2018). The HSV images need image preprocessing to obtain kinematic representations of vocal fold movements. Such preprocessing includes the estimation of vocal fold edge detection (Mehta, Deliyski, et al., 2011; Švec et al., 2007) and creation of anterior/medial/posterior kymograms (Mehta, Zañartu, et al., 2011; Švec et al., 2007). From these kinematic representations, temporal and dimensional features are calculated including relation between vibration phase of the right vocal fold with vibration phase of the left vocal fold (phase asymmetry [PA]), relation between amplitude vibration of the right vocal fold with amplitude vibration of the left vocal fold (amplitude asymmetry [AA]), ratio of opening phase duration to closing phase duration (speed quotient), and ratio of open phase duration to total period (open quotient; Mehta, Deliyski, et al., 2011; Mehta, Zañartu, et al., 2011; Švec et al., 2007). To our knowledge, HSV has not been used to investigate Lombard-related vocal fold kinematics and is expected to contribute, together with acoustic and aerodynamic measures, to elucidate how the voices of patients with NPVH are affected by the Lombard condition.
Aims and Hypotheses
The aim of this exploratory study was to investigate voice production during and immediately after elicitation of the Lombard effect with background noise in individuals with typical voices and individuals with NPVH. A comprehensive multidimensional approach for assessing vocal function, using acoustic, aerodynamic, and vocal fold vibratory measures, was implemented to provide initial insights into the pathophysiology of NPVH. In line with the theory that individuals with NPVH have auditory–motor integration difficulties, we constructed two hypotheses. It was hypothesized that individuals with NPVH would show different responses for acoustic, aerodynamic, and HSV measures during the Lombard condition when compared against individuals with typical voices. In addition, it was also expected that individuals with NPVH would show persistence of the Lombard effect after the noise eliciting the Lombard effect was removed.
Method
Participants
Thirty-eight participants were recruited for this study: individuals with typical voices (n = 19; 10 males, 9 females) and individuals with NPVH (n = 19; 7 males, 12 females). The mean (SD) of participants' ages was 27.9 (3.6) years for the individuals with typical voices and 28.5 (3.2) years for those with NPVH. All participants in the study were assessed by a laryngologist in collaboration with a speech-language pathologist (SLP) based on case history, laryngeal videostroboscopy, aerodynamic and acoustic measures of vocal function, and a Consensus Auditory-Perceptual Evaluation of Voice (CAPE-V). Participants with typical voices were recruited if they reported no history of speech, language, or hearing disorders and if the full comprehensive assessment did not reveal any abnormalities. The mean (and standard deviation) of the CAPE-V ratings for participants with NPVH is shown in Table 1, which corresponds to a mildly deviant severity. Moreover, all participants passed a pure-tone hearing screening, which consisted of positive responses to air-conduction stimuli in both ears at 20 dB HL at octave frequencies between 250 and 8000 Hz using a clinical audiometer (Model AD629, Interacoustics A/S). Participants provided written consent in accordance with the experimental protocol, which was approved by the Research and Ethics Committee of the Faculty of Medicine, Universidad de Valparaíso, Chile, based on the assessment statement Code 52015 and in compliance with the national guidelines for research with human subjects and the Declaration of Helsinki.
Table 1.
Mean (standard deviation) of Consensus Auditory-Perceptual Evaluation of Voice (CAPE-V) ratings for the participants with nonphonotraumatic vocal hyperfunction.
| CAPE-V scale | Mean (SD) rating | Severity ranking |
|---|---|---|
| Overall severity | 29.7 (11.2) | Mildly deviant |
| Roughness | 14.4 (6.6) | Mildly deviant |
| Breathiness | 11.3 (7.3) | Mildly deviant |
| Strain | 18.3 (5.7) | Mildly deviant |
| Pitch | 8.4 (5.4) | Mildly deviant |
| Loudness | 7.5 (5.3) | Mildly deviant |
Experimental Design
Participants were seated in a comfortable chair inside a sound-treated room meeting the ANSI S3.1-1999 standard in a quiet acoustic environment (less than 35 dBA, on average, air-conditioning system off). The experiment was composed of three sequential conditions: baseline (B), Lombard (L), and recovery (R). These conditions correspond to before, during, and 5 min after the Lombard condition, respectively. In each condition, participants were instructed to vocalize at a comfortable conversational pitch and loudness, with no additional instructions. Instead of a conversational task, sustained vowels and syllables were the utterances used in this study. This limited set of utterances was due to the nature of the physiological measures to be collected, which included the estimation of parameters based on the acquisition of the glottal flow and glottal pressure signals and the acquisition of laryngoscopic images. The reliable and reproducible estimation of these measures might be hampered when estimated from long vocalizations, such as those included in audiovisual Lombard grid corpus (Alghamdi et al., 2018; Marxer et al., 2018). Consequently, this study focused on the biomechanical and aerodynamic aspects of the Lombard effect rather than its communicative aspects.
In the baseline condition, participants spoke in a quiet acoustic environment. In the Lombard condition, 80 dB SPL speech-shaped noise (frequency range from 125 to 6000 Hz and −12 dB/octave above 1 kHz) generated by a clinical audiometer (Model AD629, Interacoustics A/S) was presented through closed headphones (Model 7510, RadioEar). The SPL of the speech-shaped noise used in this study is comparable with those used in previous Lombard-related studies (Junqua, 1993; Lu & Cooke, 2008; Van Summers et al., 1988), and it is loud enough to induce a robust Lombard effect while avoiding hearing discomfort and vocal/auditory fatigue (Alghamdi et al., 2018). It is worth mentioning that a certain degree of own-voice attenuation occurred due to the closed design of the headphones. Nevertheless, a significant effect of this attenuation on the acoustic measures of utterances has not been reported (Lu & Cooke, 2008). In the recovery condition, which was conducted 5 min after the speech-shaped noise was removed, participants again spoke in a quiet acoustic environment. This condition was included to allow for possible after-effects due to the noise, following previous studies (Behroozmand & Sangtian, 2018; Jones & Keough, 2008; van Brenk & Terband, 2020). The 5-min window between the Lombard and recovery conditions was selected by considering the potential for vocal fatigue due to the noise exposure. Previous studies considered speaking in noise as a vocal loading process (Fujiki & Sivasankar, 2017) and described the return to baseline for acoustic parameters as occurring within 5 min of vocal rest after noise removal in quiet conditions (Xue et al., 2019).
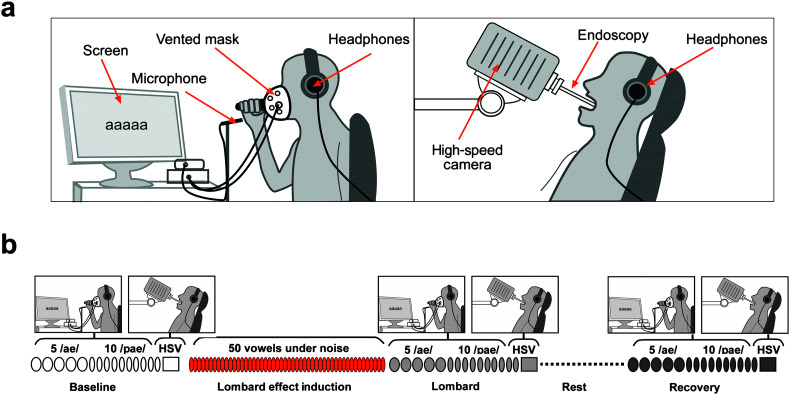
An overview of the experiments is shown in Figure 1. The acoustic, aerodynamic, and endoscopic setups are depicted in Figure 1a. Each condition (B, L, R) was recorded in two consecutive stages: Acoustic and aerodynamic signals were recorded first, followed by the endoscopic recordings, as shown in Figure 1b. Thus, participants were first asked to vocalize while wearing a circumferentially vented pneumotachograph mask (Model S/T-M1, Glottal Enterprises) and a microphone (Model 4961, B&K). The vocal gestures consisted of five repetitions of a vowels /æ/ (with a duration of 3 s, with 3 s of rest between them), followed by two sets of five consecutive /pæ/ syllables. Once this procedure was complete, the mask was removed and laryngeal HSV was performed to assess the vocal fold vibratory patterns during a single sustained vocalization of the vowel /ae/.
Figure 1.
Experimental design and procedures. (a) Diagram illustrating the experimental setup for the acquisition of acoustic and aerodynamic measures of vocal function (left panel) and laryngeal imaging using high-speed videoendoscopy (HSV; right panel). (b) Diagram representing the different stages of the experimental session. Utterances and procedures are illustrated for each experimental condition. A timescale is not presented because the length of the vocalizations and the duration of the experimental session varied between participants.
Note that the Lombard condition considered an induction to the Lombard effect (see Figure 1b, orange color), which allowed talkers to attune to the change in the acoustic environment (from quiet to noise) without inducing vocal fatigue (Alghamdi et al., 2018; Marxer et al., 2018). For this induction, participants produced 50 utterances in total, with repetitions of the vowels /æ/ and /i/. The sequence of visual cues presented on the screen was manipulated such that the vocalizations /æ/ and /i/ were performed in a pseudorandom order. For the comfort of the participants, the vented pneumotachograph mask was not worn during the Lombard induction.
Signal Acquisition and Preprocessing
The acoustic signal and the oral airflow volume velocity were simultaneously acquired during the production of the /æ/ vowels. The acoustic signal was obtained using a microphone (Model 4961, B&K) located in front of the participant at 10 cm from the lips at a 45° offset in the axial direction and amplified by a B&K 1705 signal conditioner. The acoustic signal was calibrated to physical units of dB SPL (dB re 20 μPa) using a Larson Davis calibrator (Model CAL200). The oral airflow volume velocity was acquired using an oral airflow transducer (Model PT-25-S, Glottal Enterprises) coupled to the circumferentially vented pneumotachograph mask. The oral airflow volume velocity signal was calibrated to physical units of milliliters per second using a glottal FC-1 flow calibrator. For estimating the SGP, the intraoral air pressure was acquired during the production of the two sets of five consecutive /pae/ syllables, using a PT-25-S transducer connected to a small catheter resting in the participant's oral cavity. Calibration of this signal was made with a closed-syringe system at reference levels of 0, 5, 10, 15, and 20 cm H2O using a Glottal Enterprises PC-1 pressure calibrator. All signals were sampled at 20 kHz with 16-bit quantization and low-pass filtered (3-dB cutoff frequency of 8 kHz) using a National Instruments DAQ Model USB-6363 BNC.
Postprocessing of the oral airflow volume velocity signal was performed as follows. A 10th-order Chebyshev low-pass filter with a cutoff frequency of 1.1 kHz was applied to avoid spectral distortion from the pneumotachograph mask (Rothenberg, 1973), followed by a fourth-order Butterworth high-pass filter with 3-dB cut-on frequency of 60 Hz. The intraoral pressure signal was low-pass filtered with a cutoff frequency of 80 Hz (second-order Butterworth filter). The filtering process was performed using a zero-phase approach to avoid phase distortion of the signal at the output.
Vocal SPL and Aerodynamic Measures
The vocal SPL (window size: ~80 ms) of each vocalization was computed from the full-bandwidth acoustic signal. From the oral airflow volume velocity signal, ACFL, MFDR, and OQf were estimated for each utterance. The SGP was estimated from averaging consecutive peaks of the intraoral air pressure plateaus during the middle three /p/ consonants for each of the two consecutive series for each participant, resulting in six averaged values for each of the three experimental conditions (B, L, and R). The visual representation of these aerodynamic measures is shown in Supplemental Figures S1 and S2. The mean SPL, ACFL, MFDR, OQf, and SGP across six vowel tokens were computed for each experimental condition for each participant.
The values of vocal SPL and aerodynamic measures of vocal function can significantly vary between individuals when they speak in the same acoustic environment. Consequently, we followed the approach described by Garnier et al. (2010) and Stowe and Golob (2013), to analyze SPL changes resulting from uttering in different acoustic conditions. In other words, we calculated the difference between the mean SPL computed in the Lombard condition and the baseline condition (L − B), for each participant. Likewise, the difference between the mean value in the recovery condition and baseline condition was obtained (R − B) for each participant. The same type of operation was carried out for each of the aerodynamic measures.
Note that ACFL, MFDR, OQf, and SGP were normalized (per token, then averaged per condition for each participant) with respect to SPL given the known positive correlation between aerodynamic measures and SPL (Espinoza et al., 2017; Holmberg et al., 1988, 1995). Furthermore, ACFL, MFDR, and SGP were log-transformed before normalization (dividing SPL by the 20 log10 of each measure) due to the known linear relationship between the log-scaled measures and SPL (Espinoza et al., 2017) and renamed using prime notation, namely, ACFL', MFDR', and SGP'. When normalized, aerodynamic measures show improved performance in differentiating typical and pathological vocal functions (Espinoza et al., 2017), which thus facilitates comparisons across experimental conditions and participants. Log-scaling was not necessary for OQf because of its percent-based nature.
HSV Images
The vibratory pattern of the vocal folds was captured at 8,000 frames per second with a high-speed video camera (Model FastCAM SAX2, Photron) connected to a 70° rigid oral endoscope (Model 9106, PENTAX), with a 35-mm C-mount lens adapter (lens coupler 9117, PENTAX) coupled to a 300-W xenon of source light (7152B, KayPentax). For each video, an area of interest of 350 × 300 pixels with 3,000 frames of length was manually selected by an expert SLP, with a clear and complete view of the vocal folds. The samples were initially preprocessed with video-editing software (Photron FastCAM Viewer 1.0) to optimize the level of brightness and color between the glottis and the vocal folds.
A custom MATLAB script was used to create kymograms. In the first step, an automatic algorithm to reduce translational motion artifacts was used (Mehta, Deliyski, et al., 2011). In a second step, the glottal midline was defined interactively by the end point at the anterior commissure glottis and the posterior end of the membranous glottis on the first HSV image. All images were rotated such that the glottal midline was oriented vertically. Then, an automatic edge detection and segmentation were used for obtaining the glottal edge for the left and right vocal folds. The glottis was divided into three portions, namely, posterior, middle, and anterior. A digital kymogram was created for each portion of glottis following the methods as described by Mehta, Deliyski, et al. (2011). From each kymogram, we tracked the edges of both vocal folds to obtain lateral displacement waveforms for the left and right vocal folds. Then, the left–right AA and the left–right PA were calculated. In addition, open quotient (OQw) and speed quotient were estimated from the glottal width at three different portions of the glottis (anterior, middle, and posterior; for additional methodological details, see Mehta, Deliyski, et al., 2011). The visual representation of the kymograms and associated measures is shown in Supplemental Figures S3 and S4.
Statistical Analysis
Due to the exploratory nature of this study, the relationship among the different variables characterizing the Lombard effect was not considered in the statistical model. Consequently, analyses of variance (ANOVAs) were conducted to study the effect of the group and the experimental condition for each measure. Future studies, which should include a larger sample size, may conduct multivariate ANOVAs for an accurate representation of the Lombard effect considering the dependence between measures.
Five mixed-design ANOVAs were performed to analyze the changes of the acoustic (SPL) and aerodynamic (ACFL, MFDR, OQf, and SGP) measures as a function of the experimental condition, the participant group, and the interaction. In the analysis, group was defined as the categorical factor; and the difference between conditions, the within-participant factor. The group factor had two levels: individuals with NPVH or individuals with typical voices. The factor difference between conditions had two levels: Lombard minus baseline (L − B) and recovery minus baseline (R − B). Furthermore, four mixed-design ANOVAs were performed on each SPL-normalized aerodynamic measure (ACFL', MFDR', OQf', and SGP'), with group as the categorical factor and experimental condition as the within-participant factor, and their interactions. As before, the group factor had two levels: individuals with NPVH or individuals with typical voices. The experimental condition factor had three levels: B, L, and R. Finally, mixed-design ANOVAs were also conducted for the HSV-based measures (OQw, SQw, AA, and PA) as a function of group, the glottal region, the experimental condition, and all the interactions. As in previous analyses, the group factor was set as the categorical predictor and had two levels: individuals with NPVH or individuals with typical voices. Both glottal region and experimental condition were set as within-participant factors. The glottal region factor had three levels: anterior, middle, and posterior. The same number of levels was set for the condition factor: B, L, and R.
When the factors considered in the ANOVAs had a statistically significant effect, post hoc Tukey's honestly significant difference tests were conducted. An α level of .05 was used for all significance testing. This approach corrects for the familywise error rate and is well suited for pairwise comparisons in our exploratory study. For comparisons of two group means, independent-samples, one-tailed t tests were implemented. After t tests, Hedges' g effect sizes were computed to handle any inherent bias in small sample sizes.
Results
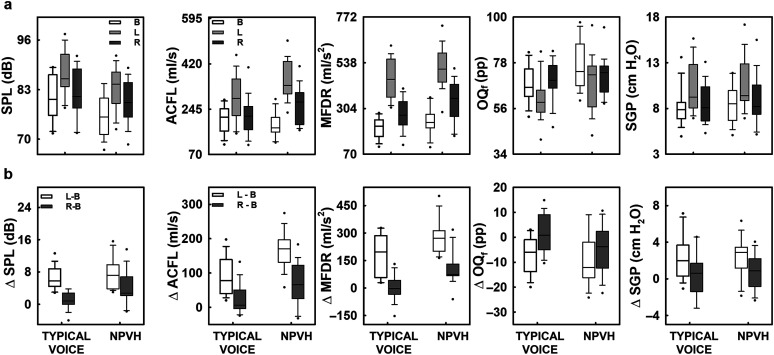
The results for vocal SPL and aerodynamic measures (ACFL, MFDR, OQf, and SGP) are presented in Figure 2. Herein, box plots show the means, the 5th, 25th, 75th, and 95th percentiles, and the outliers for each group of participants (participants with typical voices and individuals with NPVH) in the three experimental conditions (B, L, and R), as well as the differences between conditions (L − B and R − B). Descriptive statistics (means and standard deviations) are also reported in Supplemental Table S1. The results of the ANOVAs for these five measures are shown in Table 2.
Figure 2.
Acoustic and aerodynamic measures of the vocal function. (a) The behavior of the aerodynamic parameters as a function of the experimental condition: baseline (B), Lombard (L), and recovery (R). From left to right: vocal intensity (sound pressure level [SPL]), unsteady peak-to-peak airflow amplitude (ACFL), maximum flow declination rate (MFDR), flow-based laryngeal open quotient (OQf), and subglottal pressure (SGP). (b) Differences were denoted with Δ and computed between Lombard and baseline (L–B) and between recovery and baseline (R–B). Each box plot represents the mean (horizontal), the 25th and 75th percentiles (bounds of the box), and the 5th and 95th percentiles (whiskers). Outliers are presented for each group of participants (participants with typical voices and individuals with nonphonotraumatic vocal hyperfunction [NPVH]) in the different experimental conditions.
Table 2.
Summary of the mixed-design analysis of variance for the acoustic and aerodynamic measures.
| Measure | ΔSPL |
ΔACFL (ml/s) |
ΔMFDR (L/s2) |
ΔOQf (pp) |
ΔSGP (cm H2O) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Effect | F | p | F | p | F | p | F | p | F | p |
| Group (G) | 3.83 | .06 | 18.65 | .001* | 15.60 | .042* | 2.11 | .15 | 0.06 | .81 |
| Diff between conditions (DC) | 102.00 | 1.5e-5* | 68.68 | .002* | 124.50 | .021* | 14.38 | .001* | 30.15 | .004* |
| G × DC interaction | 7.90 | .028* | 0.29 | .59 | 0.49 | .59 | 1.89 | .17 | 0.07 | .79 |
Note. The effect of group (categorical factor), the difference between conditions (Diff between conditions, within-participant factor), and the interaction between factors (G × DC interaction) are presented. Statistically significant effects are denoted by asterisks (*; α = .05). The degree of freedom for the F tests was 1. SPL = sound pressure level; ΔACFL= difference in peak-to-peak airflow; ΔMFDR = difference in maximum flow declination rate; ΔOQf = difference in flow-based open quotient; ΔSGP = difference in subglottal air pressure.
Overall, the Lombard effect was evident in both participants with typical voices and individuals with NPVH, with the vocal SPL of both groups of participants increased from baseline to the Lombard condition (see Figure 2 for details). In comparison to the Lombard condition, the vocal SPL of both groups of participants decreased during the recovery condition. However, these changes did vary by group. The ANOVA revealed that the difference in SPL (ΔSPL) was significantly affected by group, condition, and their interaction. Both groups displayed a similar increase in the vocal SPL because of the Lombard effect: The ΔSPL between Lombard and baseline (L − B) did not significantly differ between groups (see Figure 2b; Tukey post hoc test, p = .79). In both groups, L − B resulted in greater ΔSPL than the difference between recovery and baseline (R − B). Furthermore, the difference R − B did not significantly differ between groups (Tukey post hoc test, p = .147). However, an independent-samples, one-tailed t test comparing the ΔSPL in the R − B conditions found that this contrast was significantly smaller in participants with typical voices than in individuals with NPVH (t = −2.83, p = .007; g = 0.94).
The interaction between group and condition did not have a statistically significant effect on any of the direct aerodynamic measures we studied (see Table 2). However, the ANOVA revealed that group and condition were statistically significant factors for ΔACFL and ΔMFDR (see Table 2). The contrast L − B was significantly higher than the contrast R − B in each group of participants (Tukey post hoc test, p = .001 and p = .042, respectively). In the case of ΔOQf and ΔSGP, only condition was the statistically significant factor (Tukey post hoc test, p = .001 and p = .004, respectively).
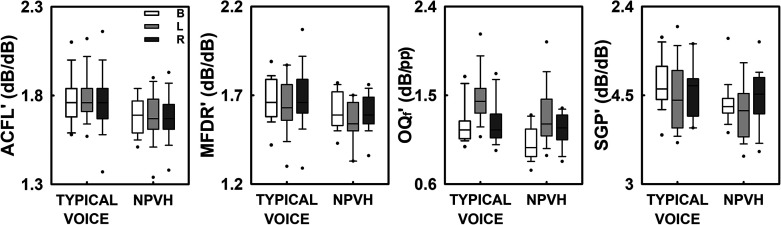
Note that SPL-normalized aerodynamic measures have been used to differentiate between matched controls and patients (Espinoza et al., 2017, 2020). Given the exploratory nature of this study, we also used these features to compare conditions to elucidate pathophysiological voice mechanisms. The results of the SPL-normalized aerodynamic measures as a function of the group and the experimental condition are illustrated in Figure 3, and Table 3 shows the results of the ANOVAs for these measures. Like the direct aerodynamic measures, none of the SPL-normalized aerodynamic measures studied showed a statistically significant effect of the interaction between group and condition. All parameters, except SGP', showed a significant effect of group: The mean ACFL', MFDR', and OQf' were all higher in individuals with typical voices than in individuals with NPVH. Furthermore, except for ACFL', the rest of SPL-normalized aerodynamic measures showed a statistically significant effect of experimental condition. Both MFDR' and SGP' decreased from baseline to Lombard and increased from Lombard to recovery. OQf' displayed the opposite behavior; this parameter increased from baseline to Lombard and decreased from Lombard to recovery. Descriptive statistics (means and standard deviations) for these measures are reported in Supplemental Table S2.
Figure 3.
Normalized aerodynamic measures of the vocal function as a function of the experimental condition: baseline (B), Lombard (L), and recovery (R). From left to right, we illustrate the normalized unsteady peak-to-peak airflow amplitude (ACFL'), the normalized maximum flow declination rate (MFDR'), the normalized flow-based laryngeal open quotient (OQf'), and the normalized subglottal pressure (SGP'). Each box plot represents the mean (horizontal), the 25th and 75th percentiles (bounds of the box), and the 5th and 95th percentiles (whiskers). Outliers are presented for each group of participants (participants with typical voices and individuals with nonphonotraumatic vocal hyperfunction [NPVH]) in the different experimental conditions.
Table 3.
Summary of the mixed-design analysis of variance for SPL-normalized aerodynamic measures.
| Measure | ACFL' (dB/dB) |
MFDR' (dB/dB) |
OQf' (dB/pp) |
SGP' (dB/dB) |
||||
|---|---|---|---|---|---|---|---|---|
| Effect | F | p | F | p | F | p | F | p |
| Group (G) | 5.79 | .021* | 5.54 | .024* | 6.95 | .012* | 3.82 | .06 |
| Condition (C) | 0.28 | .75 | 3.31 | .040* | 32.80 | 1.4e-6* | 5.95 | .004* |
| G × C interaction | 0.00 | .69 | 0.46 | .63 | 2.63 | .08 | 2.28 | .11 |
Note. The effects of group (categorical factor), condition (baseline, Lombard, and recovery; within-participant factor), and the interaction between factors (G × C interaction) are presented. Statistically significant effects are denoted by asterisks (*; α = .05). The degree of freedom for the F tests was 1. ACFL' = peak-to-peak airflow; MFDR' = normalized maximum flow declination rate; OQf' = normalized flow-based open quotient; SGP' = normalized subglottal air pressure.
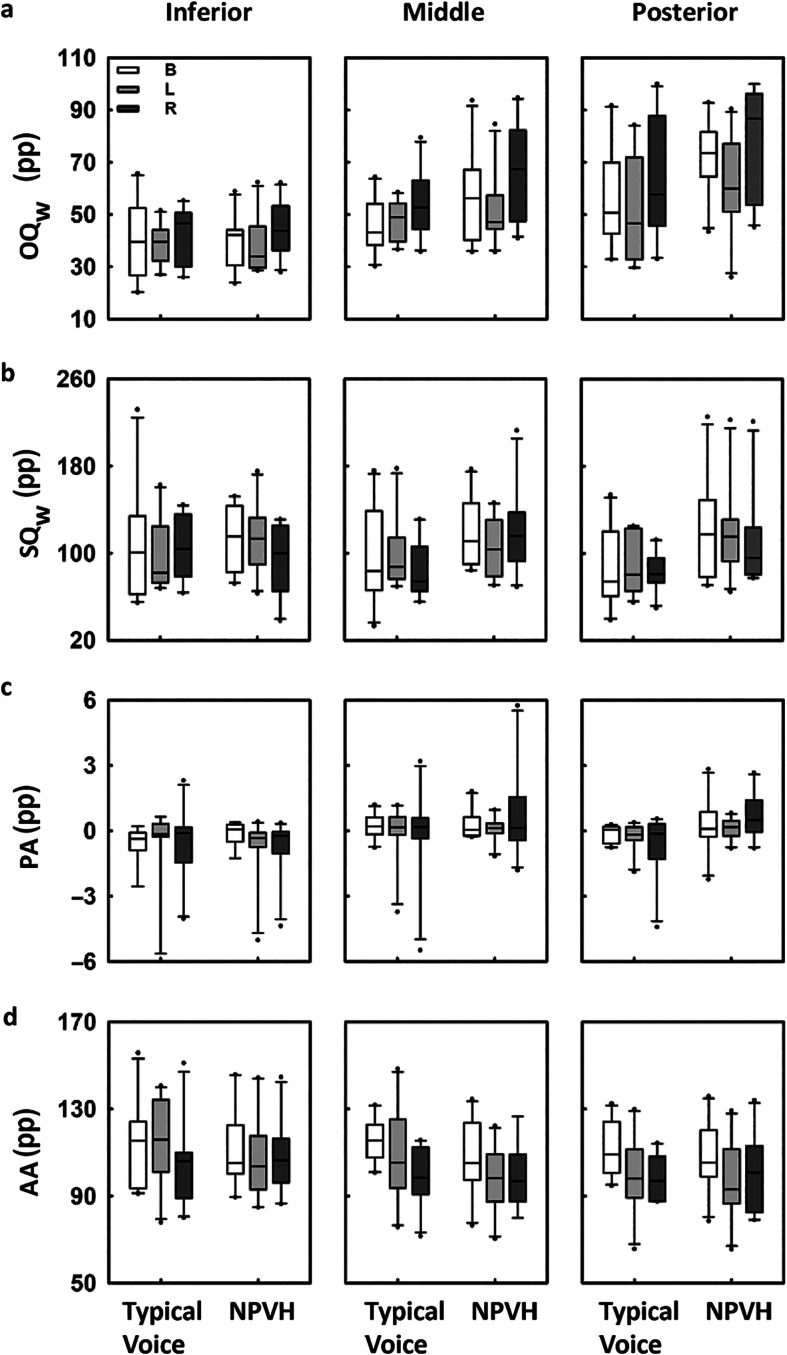
Finally, vibratory behavior of the vocal folds assessed with HSV is presented in Figure 4, and the results of the ANOVAs are shown in Table 4. The findings for OQw were generally consistent with the findings for OQf. Glottal region, condition, and their interaction were statistically significant factors for OQw. Although the OQw in both the posterior and middle regions of the glottis decreased from baseline to Lombard and increased from Lombard to recovery, the OQw of the anterior glottal region did not vary across conditions. These differences are associated with the incomplete glottal closure exhibited by both vocally typical participants and participants with NPVH. No other vibratory measure showed any statistically significant main effects or interactions. Descriptive statistics (means and standard deviations) for these measures are reported in Supplemental Table S3.
Figure 4.
Vibratory measures of vocal function obtained from high-speed videoendoscopy as a function of the experimental condition: baseline (B), Lombard (L), and recovery (R). From top to bottom, the rows show the results for width-based open quotient (OQw), width-based speed quotient (SQw), left–right phase asymmetry (PA), and left–right amplitude asymmetry (AA), all for three anterior posterior positions (inferior, middle, and posterior). Each box plot represents the mean (horizontal), the 25th and 75th percentiles (bounds of the box), and the 5th and 95th percentiles (whiskers). Outliers are presented for each group of participants (participants with typical voices and individuals with nonphonotraumatic vocal hyperfunction [NPVH]) in the different experimental conditions.
Table 4.
Summary of the mixed-design analysis of variance vibratory measures from high-speed videoendoscopy.
| Measure | OQw
|
SQw
|
PA |
AA |
||||
|---|---|---|---|---|---|---|---|---|
| Effect | F | p | F | p | F | p | F | p |
| Group (G) | 3.70 | .07 | 3.38 | .08 | 5.89 | .56 | 8.32 | .76 |
| Glottal region (GR) | 19.47 | 1.7e-5* | 0.29 | .75 | 21.14 | .74 | 3.98 | .87 |
| GR × G interaction | 1.48 | .24 | 2.74 | .08 | 2.31 | .35 | 5.43 | .21 |
| Condition (C) | 5.41 | .009* | 1.08 | .40 | 7.54 | .21 | 7.32 | .41 |
| C × G interaction | 0.53 | .59 | 0.16 | .85 | 1.21 | .93 | 8.34 | .35 |
| C × GR interaction | 2.86 | .030* | 0.09 | .98 | 2.87 | .78 | 5.74 | .75 |
| C × G × GR interaction | 0.51 | .73 | 0.85 | .49 | 0.53 | .75 | 5.42 | .91 |
Note. The effects of group (categorical factor), glottal region (anterior, middle, and posterior; within-subject factor), condition (baseline, Lombard, and recovery; within-subject factor), and the corresponding interactions are presented. Statistically significant effects are denoted by asterisks (*; α = .05). The degree of freedom for the F tests was 1. OQw = width-based open quotient; SQw = width-based speed quotient; PA = phase asymmetry; AA = amplitude asymmetry.
Discussion
In this study, we measured the variations in the vocal function (acoustic, aerodynamic, and vocal fold vibratory parameters) of individuals with typical voices and individuals with NPVH, under three conditions: speaking in a quiet environment (baseline condition), speaking under masking noise (Lombard condition), and speaking after 5 min of rest in a quiet environment (recovery condition). These conditions were selected to explore how speaking in noise affects phonation and to describe a potential persistence of the Lombard effect after noise exposure. The SPL-normalized aerodynamic measures were used in this experiment because they provide a possible way to explore what impact the Lombard effect had on the efficiency in converting the aerodynamic energy into acoustic output both during and after the Lombard effect is elicited (i.e., are there after-effects?).
Speaking in Noise
Our results showed that both groups generated a compensatory response to masking noise, with an increase in their SPL compared to their baseline condition (in a quiet environment). The participants with typical voices increased their SPL by a mean of 6.4 dB when speaking in noise; this variation is concordant with previous studies of the Lombard effect in which the experimental condition used masking noise with higher spectral energy on “speech frequencies” such as bandpass noise masker (0.5–4.0 kHz), speech noise masker (100 Hz to 4000 kHz), and broadband noise (0.2–20 kHz) at 75, 80, and 90 dB, respectively, sent by headphones (Garnier et al., 2010; Grillo et al., 2010; Meekings et al., 2016; Stowe & Golob, 2013). In addition, the mean variation in SPL is similar to that reported in previous studies when the speakers voluntarily produced louder-than-normal phonatory tasks (Espinoza et al., 2017; Holmberg et al., 1988; Sapienza & Stathopoulos, 1994; Sundberg et al., 2005).
The participants with NPVH increased their SPL by a mean of 7.3 dB when speaking in noise; this is slightly more than the participants with typical voices (but was not a statistically significant difference). As was the case for the participants with typical voices, the Lombard-related variation in SPL for participants with NPVH was similar to that reported in previous studies for voluntary changes between comfortable and loud phonation (Espinoza et al., 2017). The acoustic variations of voice due to the Lombard effect in both groups of participants were accompanied by significant increases in MFDR, ACFL, and SGP. These changes suggested an increase in the vibratory amplitudes and closure velocities of the vocal folds. Moreover, the participants with typical voices and NPVH showed a decrease in OQf, which suggested an increase in the relative duration of vocal fold closure when speaking louder in noise. These aerodynamic variations were similar to those reported in previous studies in loud conditions (Espinoza et al., 2017; Holmberg et al., 1988; Sundberg et al., 2005). In addition, the vibratory measures from HSV showed a decrease in OQw for the posterior kymogram during the Lombard condition. This finding agrees with the decrease in the aerodynamic OQf and further suggests the increase in glottal closure.
The SPL-normalized aerodynamic measures are used in this experiment because they provide a possible way to explore what impact the Lombard effect had on how the Lombard effect affected the efficiency in converting aerodynamic power into acoustic power. Previous studies demonstrated that these SPL-normalized aerodynamic measures were useful to identify differences between individuals with typical voices and individuals with NPVH (Espinoza et al., 2017). During the Lombard effect, both groups of participants displayed higher OQf' than that of the baseline condition (see Figure 4). This behavior indicates that participants become more efficient for this measure during the Lombard condition. Variations of vocal efficiency when speakers voluntarily increase SPL have been reported in previous studies (Espinoza et al., 2017) and indicate that louder voicing, either intentionally or unintentionally, is generally more efficient for the aerodynamic measures.
Our results suggest the Lombard-related increase in vocal SPL is associated with similar variations in acoustic, aerodynamic, and vibratory measures for both groups of participants. It is important to note that the Lombard effect was still reliably elicited, although the communicative environment was greatly simplified/limited (production of short monosyllables and absence of visual contact with a communication partner). In fact, the variation of SPL we observed is similar to that obtained in studies using running speech or linguistic corpus (Garnier et al., 2010; Patel & Schell, 2008; Stowe & Golob, 2013). This suggests that a minimum communicative intention is needed to elicit the Lombard effect and that the quality and complexity of the message is a secondary element in this adaptive behavior. This is supported by evolutionary studies demonstrating the widespread distribution of the Lombard effect in vertebrates, present in large numbers of species that do not have complex oral communication (for a review, see Luo et al., 2018). Moreover, as is evident from neuro-anatomic studies in different animal species, the neural circuit involved in the generation of the Lombard effect is mainly located in subcortical structures and modulated by cortical processes (Luo et al., 2018).
After-Effect of Speaking in Noise
Several studies have analyzed vocal and articulatory aspects of speech associated with the sensorimotor adaptation to the long-term presentation of auditory feedback perturbation, specifically pitch perturbation (Behroozmand & Sangtian, 2018; Jones & Keough, 2008). Consistently, these studies have reported the persistence of adaptive speech behaviors for a short time after the sensory feedback alterations were removed. In some of these studies, the persistence of the adaptive response lasted for 6 min or more (e.g., Behroozmand & Sangtian, 2018; Jones & Keough, 2008). To the best of our knowledge, the persistence of the Lombard effect has not been formally studied. Nevertheless, even some Lombard studies that were not designed to investigate after-effects have shown that, after long-term exposition to noise, the vocal SPL of some individuals remains relatively elevated even when the level of the acoustic noise is markedly decreased (Lindstrom et al., 2011).
In our study, the recovery condition was used to explore the persistence of the Lombard effect. The results showed that individuals with typical voices returned to baseline conditions for acoustic, aerodynamic, and vibratory measures after 5 min of rest. On the other hand, participants with NPVH appeared to show a persistence of the Lombard effect 5 min after removing the noise, as evidenced by a significantly greater difference in SPL in the R − B contrast compared to the individuals with typical voices. Although the aerodynamic measures ACFL, MDFR, SGP, and OQf did not return exactly to baseline values after removing the noise for individuals with NPVH, these differences were not statistically significant.
The apparent persistence of the Lombard effect for patients with NPVH raises suspicion of an underlying auditory–motor deficit. In addition, the associated persistence of an increase in laryngeal forces to maintain increased SPL after exposure to environmental noise in daily life could contribute to the vocal fatigue commonly experienced by individuals with NPVH. However, ambulatory voice monitoring studies of patients with NPVH and matched controls have not shown significant differences in daily vocal SPL that would support this hypothesis (Van Stan et al., 2021). Applying the insights gained through models of speech motor control (Houde & Nagarajan, 2011; Parrell et al., 2019; Tourville & Guenther, 2011) to our results, the persistence of the Lombard effect may reflect an update of the feedforward system for motor control of voice. When individuals speak in noise, the prediction of their voice intensity (the feedforward plan) does not match the needs associated with the real-time information from their auditory feedback due to the higher noise environment. In response, motor corrective commands are produced, increasing the intensity of their voices. Over time, speakers adapt to the new acoustic environment, generating new predictions about their intensity needs (updating the feedforward plan). Thus, when the noise is removed (the acoustic environment is quiet again), the prediction of the new feedforward command again does not match their intensity needs, and time is necessary to reestablish appropriate intensity control. Our results showed that the 5 min of rest is sufficient for updating the feedforward plans in individuals with typical voices. However, for the individuals with NPVH, this resting time was not sufficient for updating their feedforward plans, thus maintaining higher levels of SPL, ACFL, and MFDR as well as lower values of OQf even when the noise was removed.
Possible difficulties in updating feedforward commands in individuals with vocal hyperfunction have been reported in a previous study using an adaptive shift-up perturbation paradigm with a maximum perturbation of 100 cents (Stepp et al., 2017). In that study, the group of participants with hyperfunctional voice disorders exhibited more variability and typical adaptive responses to the pitch-shift alteration in comparison to participants with typical voices, either overcompensating in the opposite direction of the perturbation or “following” in the same direction of the perturbation. In addition, when the auditory feedback perturbation was removed, individuals with hyperfunctional disorders did not return to the initial pitch conditions. Whereas the pitch-shift paradigm is a direct alteration of auditory feedback of the speaker and the Lombard effect is an alteration of the speaker's environment, both approaches suggest that individuals with hyperfunctional disorders could have difficulties reprogramming and updating the feedforward commands for voice control.
Clinical Implication and Future Directions
As already noted, the apparent persistence of the Lombard effect in patients with NPVH suggests the possibility of a vocal auditory–motor deficit involving a delay or failure in updating feedforward mechanisms, which could play a role in the etiology of NPVH, that is, contribute to patients with NPVH appearing to “get stuck” in aberrant phonatory patterns (Hillman et al., 2020). If these findings can be further corroborated and refined, they could have a significant impact on the clinical management of patients with NPVH. For example, screening for auditory–motor deficits could be added to the routine clinical assessment of patients with NPVH and used as a basis for determining which patients might benefit from including auditory training in their voice therapy treatment plan. It is also possible that assessing noise levels in the daily environments of patients with NPVH could become warranted as the level and type (spectral characteristics) of noise could contribute to triggering and/or maintaining aberrant phonatory adjustments in patients with NPVH. An important topic for future studies would be to further explore the impact of different temporal windows for the Lombard and recovery conditions to assess the recovery time to return to the baseline condition in individuals with NPVH. In addition, future studies may explore how vocal training using targets in noise may help to facilitate a faster return to baseline voice characteristics when noise is removed. This may have important clinical implications, due to the large numbers of individuals working in noisy environments.
The results of our study contribute to understanding how the acoustic environment impacts the aerodynamic, acoustic, and vibratory parameters of voice production in individuals with typical voices and patients with NPVH. The results first suggest that speaking under masking noise triggers involuntary changes in the glottal configuration that increase loudness and SGP, although in an efficient manner. We highlight that the patients with NPVH maintain this elevated but efficient vocal behavior even after the removal of noise and in a resting stage. This finding can be interpreted in two ways. Background noise can lead to a persistent elevated vocal effort in patients with NPVH that is sustained even when the noise is removed, thus becoming a potential factor for vocal fatigue. On the other hand, background noise did help patients with NPVH to increase their loudness and efficiency, which is why masking noise has been described as an approach for “facilitating” improved voice production in patients with NPVH (Boone & McFarlane, 1994). In the same vein, a clinical evaluation of the auditory feedback and feedforward command could be considered for the treatment of hyperfunctional voice disorders, including evaluation of auditory acuity and audio-motor integration. The intensity and spectral content of acoustic environment where individuals carry out their daily activities may be considered in the clinical assessment of these voice disorders too.
Limitations
It is important to consider that our experimental design used a repetition of isolated sustained vowels and syllables because these gestures are required for the correct estimation of aerodynamic measures. However, this vocal task is not associated with normal communicative intention. Previous studies have described that the Lombard effect has an important communicative component and propose that the Lombard effect depends on the linguistic context and the communicative interaction (Garnier et al., 2010; Patel & Schell, 2008). It is not clear if the results of this study would generalize to more normal communicative situations (e.g., it is possible that the observed effects could be amplified by such situations), but this should be explored in future work. Note that our experiments used 5 min of rest between the Lombard and recovery conditions to explore the after-effect of speaking in noise. However, further investigating the amount of recovery time needed for individuals with NPVH to update the motor commands after the Lombard effect is necessary to better determine the clinical implications of this phenomenon.
For the assessment of the vibratory behavior from HSV, we utilized digital kymography estimated from three glottal anterior–posterior portions. For each portion, open and speed quotient as well as asymmetry measures were calculated. However, we did not find statistical significance to compare the differences among conditions. This could be due to either a lack of effect or the use of kymography not reflecting appropriately the variation produced by the Lombard effect. Future studies could explore other types of analysis such as phonovibrograms or others that provide access to the whole glottal edge. On the other hand, the HSV used a reduced temporal window analysis, and the length of video is significantly smaller than that used for estimating other measures. This may have affected the accuracy of the HSV analysis.
Conclusions
This study explored voice production during the Lombard effect and its persistence when the masking noise was removed in volunteers with typical voices and those with NPVH. Our results show that speaking under noise elevates acoustic and aerodynamic measures without changing the symmetry in the vibration. Moreover, after 5 min of removing the noise, the individuals with typical voices return near to the baseline condition. In contrast, the individuals with NPVH show a difference between baseline and recovery, thus suggesting a persistence of the Lombard effect. This after-effect could be associated with disruptions to updating commands of the feedforward voice control system in these patients after the noise is removed, which could play a role in the disorder etiology. Further efforts are needed to better understand the relationship between auditory processing and laryngeal motor control production in patients with vocal hyperfunction.
Supplementary Material
Acknowledgments
This research was supported in part by Agencia Nacional de Investigación y Desarrollo through Grants FONDECYT 1191369 and BASAL FB0008 (principal investigator: Matías Zañartu) and FONDECYT 11200665 (principal investigator: Víctor M. Espinoza), the Voice Health Institute, and National Institute on Deafness and Other Communication Disorders Grant P50 DC015446 (principal investigator: Robert E. Hillman). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding Statement
This research was supported in part by Agencia Nacional de Investigación y Desarrollo through Grants FONDECYT 1191369 and BASAL FB0008 (principal investigator: Matías Zañartu) and FONDECYT 11200665 (principal investigator: Víctor M. Espinoza), the Voice Health Institute, and National Institute on Deafness and Other Communication Disorders Grant P50 DC015446 (principal investigator: Robert E. Hillman). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Abur, D. , Subaciute, A. , Kapsner-Smith, M. , Segina, R. K. , Tracy, L. F. , Noordzij, J. P. , & Stepp, C. E. (2021). Impaired auditory discrimination and auditory–motor integration in hyperfunctional voice disorders. Scientific Reports, 11(1), Article 13123. https://doi.org/10.1038/s41598-021-92250-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghamdi, N. , Maddock, S. , Marxer, R. , Barker, J. , & Brown, G. J. (2018). A corpus of audio-visual Lombard speech with frontal and profile views. The Journal of the Acoustical Society of America, 143(6), EL523–EL529. https://doi.org/10.1121/1.5042758 [DOI] [PubMed] [Google Scholar]
- Behroozmand, R. , & Sangtian, S. (2018). Neural bases of sensorimotor adaptation in the vocal motor system. Experimental Brain Research, 236(7), 1881–1895. https://doi.org/10.1007/s00221-018-5272-9 [DOI] [PubMed] [Google Scholar]
- Bonilha, H. S. , Deliyski, D. D. , Whiteside, J. P. , & Gerlach, T. T. (2012). Vocal fold phase asymmetries in patients with voice disorders: A study across visualization techniques. American Journal of Speech-Language Pathology, 21(1), 3–15. https://doi.org/10.1044/1058-0360(2011/09-0086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone, D. R. , & McFarlane, S. C. (1994). The voice and voice therapy. Prentice-Hall. [Google Scholar]
- Demmink-Geertman, L. , & Dejonckere, P. H. (2002). Nonorganic habitual dysphonia and autonomic dysfunction. Journal of Voice, 16(4), 549–559. https://doi.org/10.1016/S0892-1997(02)00130-3 [DOI] [PubMed] [Google Scholar]
- Espinoza, V. M. , Mehta, D. D. , Van Stan, J. H. , Hillman, R. E. , & Zañartu, M. (2020). Glottal aerodynamics estimated from neck-surface vibration in women with phonotraumatic and nonphonotraumatic vocal hyperfunction. Journal of Speech, Language, and Hearing Research, 63(9), 2861–2869. https://doi.org/10.1044/2020_JSLHR-20-00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza, V. M. , Zañartu, M. , Van Stan, J. H. , Mehta, D. D. , & Hillman, R. E. (2017). Glottal aerodynamic measures in women with phonotraumatic and nonphonotraumatic vocal hyperfunction. Journal of Speech, Language, and Hearing Research, 60(8), 2159–2169. https://doi.org/10.1044/2017_JSLHR-S-16-0337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki, R. B. , & Sivasankar, M. P. (2017). A review of vocal loading tasks in the voice literature. Journal of Voice, 31(3), 388.e33–388.e39. https://doi.org/10.1016/j.jvoice.2016.09.019 [DOI] [PubMed] [Google Scholar]
- Galindo, G. E. , Peterson, S. D. , Erath, B. D. , Castro, C. , Hillman, R. E. , & Zañartu, M. (2017). Modeling the pathophysiology of phonotraumatic vocal hyperfunction with a triangular glottal model of the vocal folds. Journal of Speech, Language, and Hearing Research, 60(9), 2452–2471. https://doi.org/10.1044/2017_JSLHR-S-16-0412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier, M. , & Henrich, N. (2014). Speaking in noise: How does the Lombard effect improve acoustic contrasts between speech and ambient noise? Computer Speech & Language, 28(2), 580–597. https://doi.org/10.1016/j.csl.2013.07.005 [Google Scholar]
- Garnier, M. , Henrich, N. , & Dubois, D. (2010). Influence of sound immersion and communicative interaction on the Lombard effect. Journal of Speech, Language, and Hearing Research, 53(3), 588–608. https://doi.org/10.1044/1092-4388(2009/08-0138) [DOI] [PubMed] [Google Scholar]
- Grillo, E. , Verdolini, K. , & Lee, T. D. (2010). Effects of masking noise on laryngeal resistance for breathy, normal, and pressed voice. Journal of Speech, Language, and Hearing Research, 53(4), 850–861. https://doi.org/10.1044/1092-4388(2009/08-0069) [DOI] [PubMed] [Google Scholar]
- Hillman, R. E. , Holmberg, E. B. , Perkell, J. S. , Walsh, M. , & Vaughan, C. (1989). Objective assessment of vocal hyperfunction: An experimental framework and initial results. Journal of Speech and Hearing Research, 32(2), 373–392. https://doi.org/10.1044/jshr.3202.373 [DOI] [PubMed] [Google Scholar]
- Hillman, R. E. , Stepp, C. E. , Van Stan, J. H. , Zañartu, M. , & Mehta, D. D. (2020). An updated theoretical framework for vocal hyperfunction. American Journal of Speech-Language Pathology, 29(4), 2254–2260. https://doi.org/10.1044/2020_AJSLP-20-00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg, E. B. , Hillman, R. E. , & Perkell, J. S. (1988). Glottal airflow and transglottal air pressure measurements for male and female speakers in soft, normal, and loud voice. The Journal of the Acoustical Society of America, 84(2), 511–529. https://doi.org/10.1121/1.396829 [DOI] [PubMed] [Google Scholar]
- Holmberg, E. B. , Hillman, R. E. , Perkell, J. S. , Guiod, P. C. , & Goldman, S. L. (1995). Comparisons among aerodynamic, electroglottographic, and acoustic spectral measures of female voice. Journal of Speech and Hearing Research, 38(6), 1212–1223. https://doi.org/10.1044/jshr.3806.1212 [DOI] [PubMed] [Google Scholar]
- Houde, J. F. , & Nagarajan, N. S. (2011). Speech production as state feedback control. Frontiers in Human Neuroscience, 5, 82. https://doi.org/10.3389/fnhum.2011.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, J. A. , & Keough, D. (2008). Auditory–motor mapping for pitch control in singers and nonsingers. Experimental Brain Research, 190(3), 279–287. https://doi.org/10.1007/s00221-008-1473-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junqua, J.-C. (1993). The Lombard reflex and its role on human listeners and automatic speech recognizers. The Journal of the Acoustical Society of America, 93(1), 510–524. https://doi.org/10.1121/1.405631 [DOI] [PubMed] [Google Scholar]
- Kryshtopava, M. , Van Lierde, K. , Meerschman, I. , D, Haeseleer, E. , Vandemaele, P. , Vingerthoets, G. , & Claeys, S. (2017). Brain activity during phonation in women with muscle tension dysphonia: An fMRI study. Journal of Voice, 31(6), 675–690. https://doi.org/10.1016/j.jvoice.2017.03.010 [DOI] [PubMed] [Google Scholar]
- Lindstrom, F. , Waye, K. P. , Södersten, M. , McAllister, A. , & Ternström, S. (2011). Observations of the relationship between noise exposure and preschool teacher voice usage in day-care center environments. Journal of Voice, 25(2), 166–172. https://doi.org/10.1016/j.jvoice.2009.09.009 [DOI] [PubMed] [Google Scholar]
- Lombard, E. (1911). Le signe de l'élévation de la voix [The sign of voice raising]. Annales des Maladies de l'Oreille et du Larynx, 37, 101–119. [Google Scholar]
- Lu, Y. , & Cooke, M. (2008). Speech production modifications produced by competing talkers, babble, and stationary noise. The Journal of the Acoustical Society of America, 124(5), 3261–3275. https://doi.org/10.1121/1.2990705 [DOI] [PubMed] [Google Scholar]
- Luo, J. , Hage, S. R. , & Moss, C. F. (2018). The Lombard effect: From acoustics to neural mechanisms. Trends in Neurosciences, 41(12), 938–949. https://doi.org/10.1016/j.tins.2018.07.011 [DOI] [PubMed] [Google Scholar]
- Marxer, R. , Barker, J. , Alghamdi, N. , & Maddock, S. (2018). The impact of the Lombard effect on audio and visual speech recognition systems. Speech Communication, 100, 58–68. https://doi.org/10.1016/j.specom.2018.04.006 [Google Scholar]
- McKenna, V. S. , Hylkema, J. A. , Tardif, M. C. , & Stepp, C. E. (2020). Voice onset time in individuals with hyperfunctional voice disorders: Evidence for disordered vocal motor control. Journal of Speech, Language, and Hearing Research, 63(2), 405–420. https://doi.org/10.1044/2019_JSLHR-19-00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meekings, S. , Evans, S. , Lavan, N. , Boebinger, D. , Krieger-Redwood, K. , Cooke, M. , & Scott, S. K. (2016). Distinct neural systems recruited when speech production is modulated by different masking sounds. The Journal of the Acoustical Society of America, 140(1), 8–19. https://doi.org/10.1121/1.4948587 [DOI] [PubMed] [Google Scholar]
- Mehta, D. D. , Deliyski, D. D. , Quatieri, T. F. , & Hillman, R. E. (2011). Automated measurement of vocal fold vibratory asymmetry from high-speed videoendoscopy recordings. Journal of Speech, Language, and Hearing Research, 54(1), 47–54. https://doi.org/10.1044/1092-4388(2010/10-0026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, D. D. , Deliyski, D. D. , Zeitels, S. M. , Zañartu, M. , & Hillman, R. E. (2015). Integration of transnasal fiberoptic high-speed videoendoscopy with time-synchronized recordings of vocal function. In Izdebski K., Yan Y., & Patel R. (Eds.), Normal & abnormal vocal folds kinematics: HSDP, OCT & NBI, Volume I: Technology (pp. 105–114). Pacific Voice & Speech Foundation. [Google Scholar]
- Mehta, D. D. , Kobler, J. , Zeitels, S. , Zañartu, M. , Erath, B. D. , Motie-Shirazi, M. , Peterson, S. D. , Petrillo, R. , & Hillman, R. E. (2019). Toward development of a vocal fold contact pressure probe: Bench-top validation of a dual-sensor probe using excised human larynx models. Applied Sciences, 9(20), 4360. https://doi.org/10.3390/app9204360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, D. D. , Zañartu, M. , Quatieri, T. F. , Deliyski, D. D. , & Hillman, R. E. (2011). Investigating acoustic correlates of human vocal fold vibratory phase asymmetry through modeling and laryngeal high-speed videoendoscopy. The Journal of the Acoustical Society of America, 130(6), 3999–4009. https://doi.org/10.1121/1.3658441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison, M. D. , Rammage, L. A. , Belisle, G. M. , Pullan, C. B. , & Nichol, H. (1983). Muscular tension dysphonia. Journal of Otolaryngology, 12(5), 302–306. [PubMed] [Google Scholar]
- Motie-Shirazi, M. , Zañartu, M. , Peterson, S. D. , Mehta, D. D. , Kobler, J. , Hillman, R. E. , & Erath, B. D. (2019). Toward development of a vocal fold contact pressure probe: Sensor characterization and validation using synthetic vocal fold models. Applied Sciences, 9(15), 3002. https://doi.org/10.3390/app9153002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasir, S. M. , Darainy, M. , & Ostry, D. J. (2013). Sensorimotor adaptation changes the neural coding of somatosensory stimuli. Journal of Neurophysiology, 109(8), 2077–2085. https://doi.org/10.1152/jn.00719.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oates, J. , & Winkworth, A. (2008). Characterising hyperfunctional voice disorders: Etiology, assessment, treatment and prevention. International Journal of Speech-Language Pathology, 10(4), 193–194. https://doi.org/10.1080/17549500802140948 [DOI] [PubMed] [Google Scholar]
- Parrell, B. , Ramanarayanan, V. , Nagarajan, S. , & Houde, J. (2019). The FACTS model of speech motor control: Fusing state estimation and task-based control. PLOS Computational Biology, 15(9), e1007321. https://doi.org/10.1371/journal.pcbi.1007321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, R. , & Schell, K. W. (2008). The influence of linguistic content on the Lombard effect. Journal of Speech, Language, and Hearing Research, 51(1), 209–220. https://doi.org/10.1044/1092-4388(2008/016 [DOI] [PubMed] [Google Scholar]
- Rosenthal, A. L. , Lowell, S. Y. , & Colton, R. H. (2014). Aerodynamic and acoustic features of vocal effort. Journal of Voice, 28(2), 144–153. https://doi.org/10.1016/j.jvoice.2013.09.007 [DOI] [PubMed] [Google Scholar]
- Rothenberg, M. (1973). A new inverse-filtering technique for deriving the glottal air flow waveform during voicing. The Journal of the Acoustical Society of America, 53(6), 1632–1645. https://doi.org/10.1121/1.1913513 [DOI] [PubMed] [Google Scholar]
- Roy, N. , & Bless, D. M. (2000). Personality traits and psychological factors in voice pathology: A foundation for future research. Journal of Speech, Language, and Hearing Research, 43(3), 737–748. https://doi.org/10.1044/jslhr.4303.737 [DOI] [PubMed] [Google Scholar]
- Roy, N. , Dietrich, M. , Blomgren, M. , Heller, A. , Houtz, D. R. , & Lee, J. (2019). Exploring the neural bases of primary muscle tension dysphonia: A case study using functional magnetic resonance imaging. Journal of Voice, 33(2), 183–194. https://doi.org/10.1016/j.jvoice.2017.11.009 [DOI] [PubMed] [Google Scholar]
- Samargia, S. , Schmidt, R. , & Kimberley, T. J. (2016). Cortical silent period reveals differences between adductor spasmodic dysphonia and muscle tension dysphonia. Neurorehabilitation and Neural Repair, 30(3), 221–232. https://doi.org/10.1177/1545968315591705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapienza, C. M. , & Stathopoulos, E. T. (1994). Comparison of maximum flow declination rate: Children versus adults. Journal of Voice, 8(3), 240–247. https://doi.org/10.1016/s0892-1997(05)80295-4 [DOI] [PubMed] [Google Scholar]
- Sato, H. , & Bradley, J. S. (2008). Evaluation of acoustical conditions for speech communication in working elementary school classrooms. The Journal of the Acoustical Society of America, 123(4), 2064–2077. https://doi.org/10.1121/1.2839283 [DOI] [PubMed] [Google Scholar]
- Stepp, C. E. , Lester-Smith, R. A. , Abur, D. , Daliri, A. , Pieter, N. , & J., & Lupiani, A. A. (2017). Evidence for auditory–motor impairment in individuals with hyperfunctional voice disorders. Journal of Speech, Language, and Hearing Research, 60(6), 1545–1550. https://doi.org/10.1044/2017_JSLHR-S-16-0282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe, L. M. , & Golob, E. J. (2013). Evidence that the Lombard effect is frequency-specific in humans. The Journal of the Acoustical Society of America, 134(1), 640–647. https://doi.org/10.1121/1.4807645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg, J. , Fahlstedt, E. , & Morell, A. (2005). Effects on the glottal voice source of vocal loudness variation in untrained female and male voices. The Journal of the Acoustical Society of America, 117(2), 879–885. https://doi.org/10.1121/1.1841612 [DOI] [PubMed] [Google Scholar]
- Švec, J. G. , Šram, F. , & Schutte, H. K. (2007). Videokymography in voice disorders: What to look for? Annals of Otology, Rhinology & Laryngology, 116(3), 172–180. https://doi.org/10.1177/000348940711600303 [DOI] [PubMed] [Google Scholar]
- Tam, K. H. , Carding, P. , Heard, R. , & Madhill, C. J. (2018). The relationship between voice quality and pitch discrimination ability in a population with features of mild vocal hyperfunction. Presented at the Voice Foundation's 47th Annual Symposium, Philadelphia, PA. [Google Scholar]
- Tourville, J. A. , & Guenther, F. H. (2011). The DIVA model: A neural theory of speech acquisition and production. Language and Cognitive Processes, 26(7), 952–981. https://doi.org/10.1080/01690960903498424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Brenk, F. , & Terband, H. (2020). Compensatory and adaptive responses to real-time formant shifts in adults and children. The Journal of the Acoustical Society of America, 147(4), 2261–2270. https://doi.org/10.1121/10.0001018 [DOI] [PubMed] [Google Scholar]
- Van Stan, J. H. , Ortiz, A. J. , Cortes, J. P. , Marks, K. L. , Toles, L. E. , Mehta, D. D. , Burns, J. A. , Hron, T. , Stadelman-Cohen, T. , Krusemark, C. , Muise, J. , Fox-Galalis, A. B. , Nudelman, C. , Zeitels, S. , & Hillman, R. E. (2021). Differences in daily voice use measures between female patients with nonphonotraumatic vocal hyperfunction and matched controls. Journal of Speech, Language, and Hearing Research, 64(5), 1457–1470. https://doi.org/10.1044/2021_JSLHR-20-00538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Summers, W. , Pisoni, D. , Bernacki, R. , Pedlow, R. , & Stokes, M. (1988). Effects of noise on speech production: Acoustic and perceptual analyses. The Journal of the Acoustical Society of America, 84(3), 917–928. https://doi.org/10.1121/1.396660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittico, T. H. , Ortiz, A. J. , Marks, K. L. , Toles, L. E. , Van Stan, J. H. , Hillman, R. E. , & Mehta, D. D. (2020). Ambulatory monitoring of Lombard-related vocal characteristics in vocally healthy female speakers. The Journal of the Acoustical Society of America, 147(6), EL552–EL558. https://doi.org/10.1121/10.0001446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, C. , Kang, J. , Hedberg, C. , Zhang, Y. , & Jiang, J. J. (2019). Dynamically monitoring vocal fatigue and recovery using aerodynamic, acoustic, and subjective self-rating measurements. Journal of Voice, 33(5), 809.e11–809.e18. https://doi.org/10.1016/j.jvoice.2018.03.014 [DOI] [PubMed] [Google Scholar]
- Yamauchi, A. , Yokonishi, H. , Imagawa, H. , Sakakibara, K. , Nito, T. , Tayama, N. , & Yamasoba, T. (2016). Quantification of vocal fold vibration in various laryngeal disorders using high-speed digital imaging. Journal of Voice, 30(2), 205–214. https://doi.org/10.1016/j.jvoice.2015.04.016 [DOI] [PubMed] [Google Scholar]
- Zacharias, S. , Deliyski, D. D. , & Gerlach, T. T. (2018). Utility of laryngeal high-speed videoendoscopy in clinical voice assessment. Journal of Voice, 32(2), 216–220. https://doi.org/10.1016/j.jvoice.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zañartu, M. , Galindo, G. E. , Erath, B. D. , Peterson, S. D. , Wodicka, G. R. , & Hillman, R. E. (2014). Modeling the effects of a posterior glottal opening on vocal fold dynamics with implications for vocal hyperfunction. The Journal of the Acoustical Society of America, 136(6), 3262–3271. https://doi.org/10.1121/1.4901714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziethe, A. , Petermann, S. , Hoppe, U. , Greiner, N. , Bruning, M. , Bohr, C. , & Dollinger, M. (2019). Control of fundamental frequency in dysphonic patients during phonation and speech. Journal of Voice, 33(6), 851–859. https://doi.org/10.1016/j.jvoice.2018.07 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.