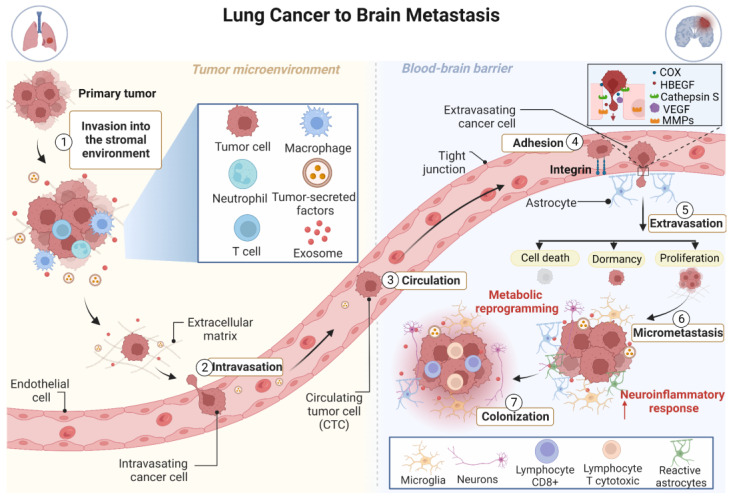
Figure 1.
Schematic illustration of brain metastasis development through hematogenous dissemination. Initially, tumor cells at the primary site acquire invasive properties, break away from the primary tumor, and invade the surrounding tissues (intravasation) becoming circulating tumor cells (CTCs). Cell motility is promoted through the interaction between tumor cells and immune cells. Then, CTCs spread throughout the circulatory system to the brain microvasculature (circulation) and after their adhesion with help of integrins, they start the extravasation across the blood–brain barrier (BBB). Tumor cells overexpress enzymes associated with mitogenesis, growth factors, metalloproteinases, and proteases allowing cell migration through the BBB. Once tumor cells are located in the central nervous system (CNS), an intense neuroinflammatory response is triggered. After extravasating, most tumor cells die or enter a state of dormancy (some of them could stay dormant at metastatic sites for long periods). Few tumor cells are able to proliferate within this new microenvironment and then form micrometastases and colonize the brain (colonization). The interaction between tumor cells and immune cells residing in the brain is critical for the establishment and growth of the tumor. COX2: prostaglandin-endoperoxide synthase 2; HBEGF: heparin-binding EGF-like growth factor; MMPs: metalloproteinases; VEGF: vascular endothelial growth factor.