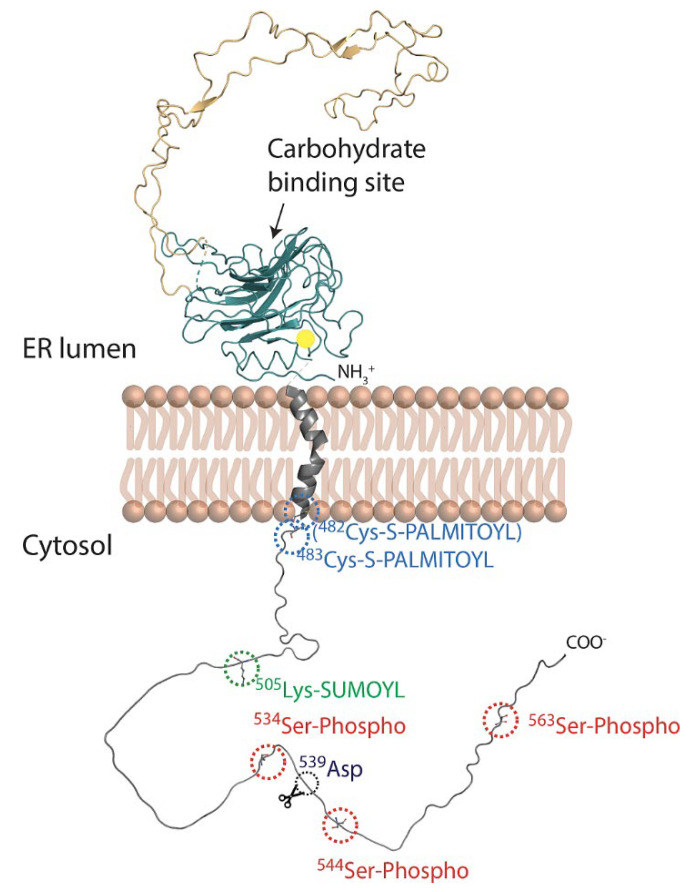
Figure 3.
Schematic representation of full-length membrane-embedded calnexin. Calnexin (Protein Data Bank DOI: 10.2210/pdb1JHN/pdb) has three domains: luminal domain (comprised of N and P subdomains), transmembrane domain and C-terminal domain (no experimental structural information available). The yellow circle depicts a bound Ca2+ ion. The numbering of amino acid residues in the C-terminal domain is relative to the mature N-terminus. The transmembrane domain was modelled based on molecular dynamics simulation performed by Lakkaraju et al. [34] and shows that Pro494 introduces a kink in the helix located approximately at the midpoint of the domain. The C-terminal domain was modelled using AlphaFold [98]. The calnexin C-terminal domain undergoes distinct post-translational modifications including palmitoylation at Cys482 and Cys483 [35] (shown in blue); sumoylation at Lys505 [96] (shown in green); and phosphorylation at Ser534, Ser544 and Ser563 [97] (shown in red); Asp539 proteolytic cleavage site (shown in black). Known and potential sites of post-translational modifications in the calnexin C-terminal domains of various species are shown in Supplementary Figure S1.