Figure 6.
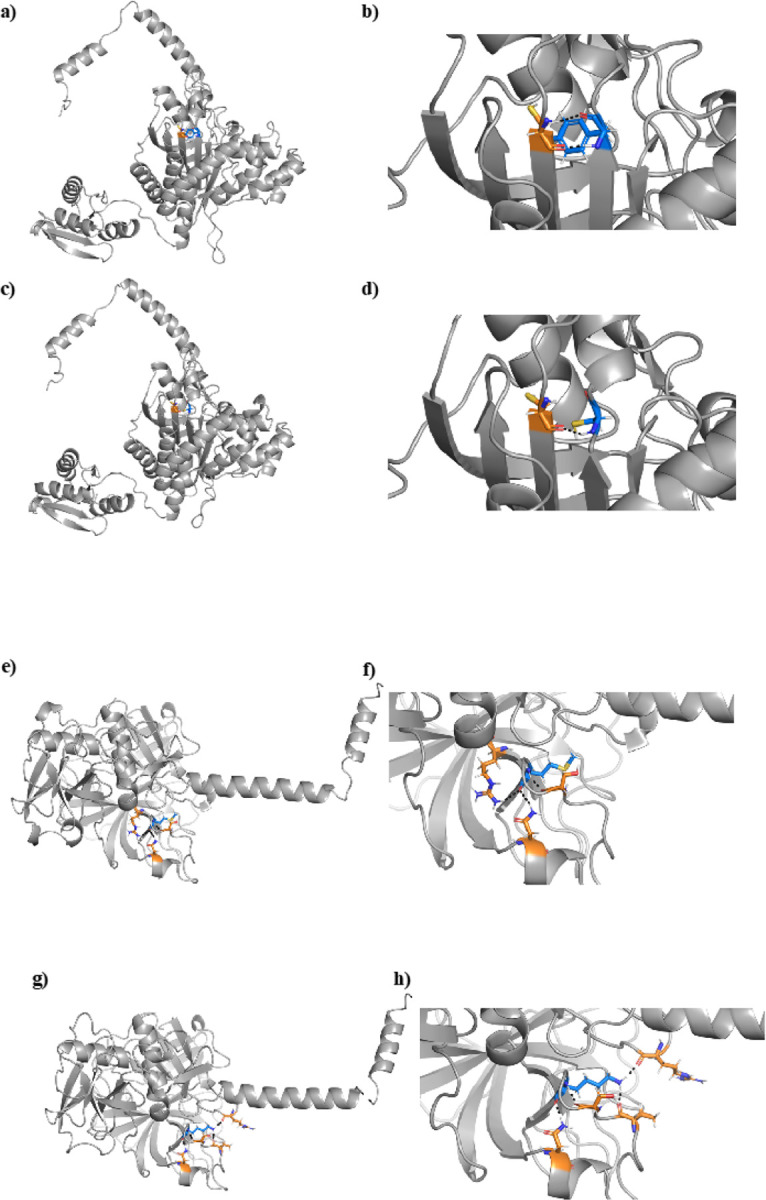
The protein structure of HARS2 variant NP_036340.1:p.Tyr364Cys (Panels A-D) and TMPRSS3 variant NP_076927.1:p.Met384Lys (Panels E-H). A) The wildtype HARS2 protein contains a tyrosine (blue) at position 364, which interacts with a neighboring cysteine amino acid (orange) B) Augmentation of the boxed region in Panel A shows two hydrogen bonds between the tyrosine and cysteine. C) The NP_036340.1:p.Tyr364Cys variant introduces a new cysteine (blue) in place of tyrosine. D) Enlargement of the boxed region from Panel C shows that the variant cysteine (blue) interacts with the original neighboring cysteine (orange), disrupting the two hydrogen bonds to form a single hydrogen bond or a disulfide bond. E) The wildtype TMPRSS3 protein shows a methionine (blue) at position 384, which interacts with three neighboring amino acids (orange). F) Magnification of Panel E shows three hydrogen bonds between the methionine and neighboring amino acids. G) The NP_076927.1:p.Met384Lys variant introduces a lysine (blue) in place of methionine, which interacts with four neighboring amino acids, only one of which remains the same as the wildtype interacting neighbors. H) Enlargement of the boxed region from Panel G shows four hydrogen bonds between the lysine and neighboring amino acids. While one hydrogen bond remains the same between the wildtype and variant structures, the NP_076927.1:p.Met384Lys variant results in significant misfolding
