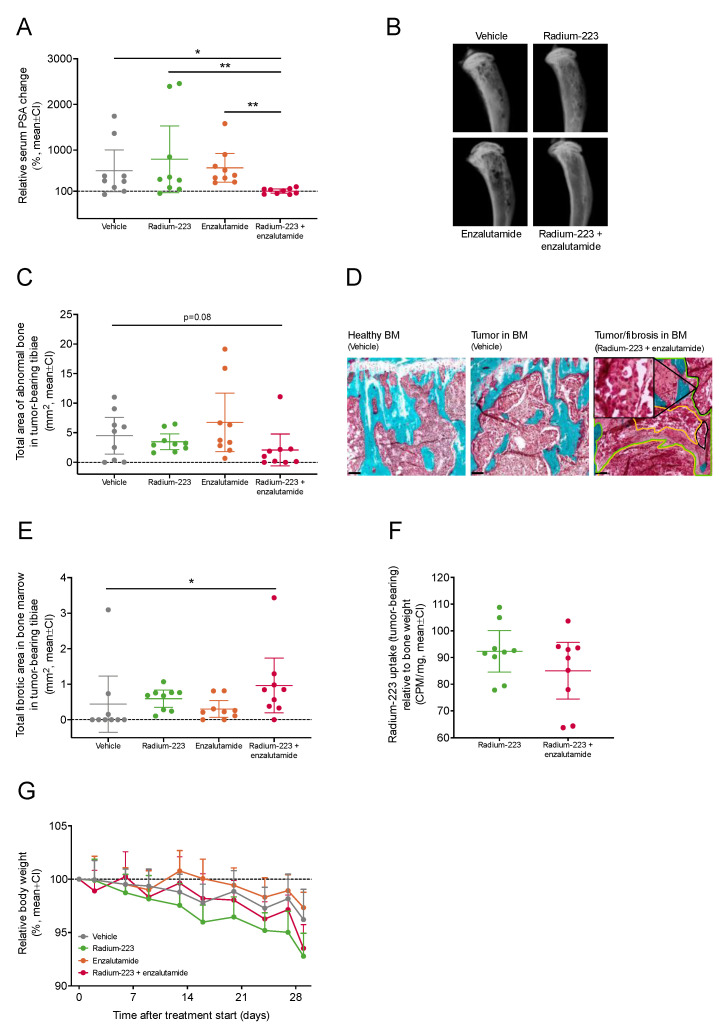
Figure 3.
Combination treatment with radium-223 and enzalutamide exhibits enhanced antitumor efficacy in vivo. Blood samples were collected from the saphenous vein of mice (n = 9/group) treated with vehicle, radium-223 (330 kBq/kg, Q4W × 2), enzalutamide (30 mg/kg, QD, p.o.) or the combination of radium-223 and enzalutamide. (A) Serum PSA change at the end of the study relative to the pre-treatment baseline. (B) Representative ex vivo X-ray images of tumor-bearing tibiae. (C) Total area of abnormal bone measured by radiography. (D) Representative histology images (10×) of healthy bone marrow (BM) and LNCaP tumor tissue in bone marrow stained with Masson-Goldner’s trichrome. Tumor (black lines, with a zoomed insert of tumor), necrotic (yellow lines) and fibrotic (green lines) tissue areas are annotated in the image on right. Scale bar length: 100 μm. (E) Total fibrotic area in the bone marrow of tumor-bearing tibiae as determined by histology. (F) Radium-223 uptake in bone determined by measuring radioactivity in the tumor-bearing tibiae. The results are expressed as counts per minute (CPM) normalized to the weight of the bone sample. Plots describe mean and 95% confidence interval (CI). (G) Relative body weights of mice treated with vehicle, radium-223, enzalutamide, or their combination. Body weights were recorded twice weekly during the treatment period The figure describes relative body weight compared to the treatment start, shown as mean and 95% confidence interval (CI). The statistical analyses were performed using ANOVA (A,C), Kruskal–Wallis test and pairwise comparisons using Dunn’s test (E), or Welch’s t-test (F): * p < 0.05; ** p < 0.01.