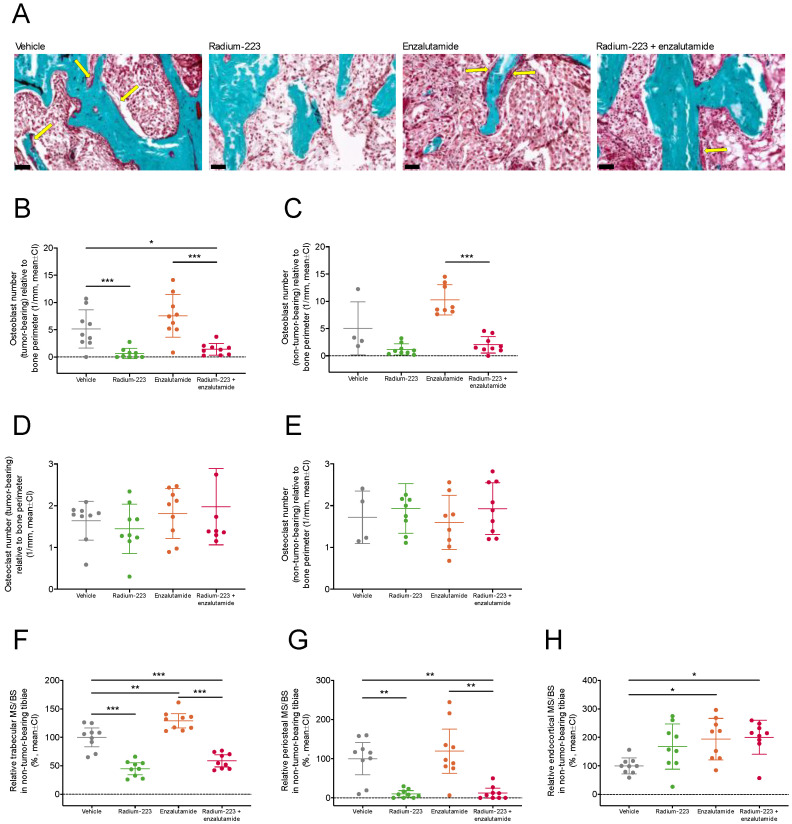
Figure 5.
Concurrent enzalutamide administration does not impair radium-223-specific antitumor effects in bone or its ability to inhibit abnormal bone formation. The mice (n = 9/group) were treated with vehicle, radium-223 (330 kBq/kg, Q4W × 2, i.v.), enzalutamide (30 mg/kg, QD, p.o.), or their combination. The bones were labeled with alizarin red and calcein green in vivo for dynamic histomorphometry. (A) Representative images (20×) of osteoblast clusters (indicated with yellow arrows) in tumor-bearing tibiae in all treatment groups visualized by Masson-Goldner’s trichrome staining. Scale bar length: 50 μm. The number of (B,C) osteoblasts and (D,E) osteoclasts relative to bone perimeter in tumor-bearing (n = 9/group) and non-tumor-bearing tibiae (n = 9/group, except vehicle, n = 4; enzalutamide, n = 8) as determined by histomorphometry. Bone formation parameters, (F) trabecular, (G) periosteal and (H) endocortical mineralizing surface per trabecular, periosteal and endocortical bone surface (MS/BS), respectively, were measured in the non-tumor-bearing tibiae by dynamic histomorphometry. Plots indicate mean and 95% confidence interval (CI). The statistical analyses were performed using ANOVA followed by contrasts, or Kruskal–Wallis test followed by Dunn’s test: * p < 0.05; ** p < 0.01; *** p < 0.001.