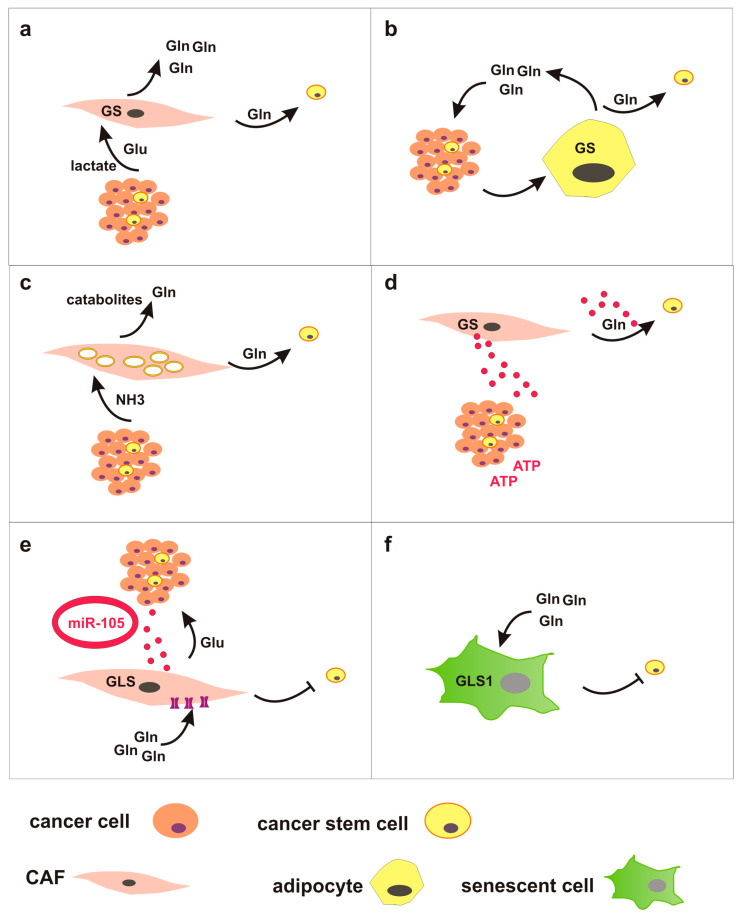
Figure 3.
TME components may locally affect glutamine concentration and availability, thereby modulating neighboring CSC. (a) Crosstalk between CAF and cancer cells in the TME. CAF upregulate GS and under glutamine-deprived conditions sustain proliferation of glutamine-dependent cancer cells. On the other side, cancer cells release lactate and glutamate to support CAF metabolism. Glutamine produced by CAF might be available for CSC. (b) Crosstalk between adipocytes and cancer cells in the TME. High glutamine demand in cancer cells results in glutamine depletion within the TME and induces GS upregulation in dysfunctional cancer-associated adipocytes. Glutamine produced by adipocytes might be available for CSC. (c) Crosstalk between CAF and cancer cells in the TME. Ammonia produced by cancer cells selectively induces autophagy in CAF, leading to release of catabolites, including glutamine, which are taken up by cancer cells. Glutamine produced by CAF might be available for CSC. (d) Exosomes-mediated crosstalk between CAF and cancer cells in the TME. CAF-derived exosomes contain metabolites, including glutamine, which can be used by cancer cells. Glutamine-containing exosomes produced by CAF might be available for CSC. (e) Exosomes-mediated crosstalk between CAF and cancer cells in the TME. Exosome-encapsulated miR-105, released by cancer cells, induces upregulation of GLS and SLC1A5 in CAF. High glutamine demand in CAF can reduce glutamine availability for CSC. (f) Senescent cells upregulate GLS1 and rely on glutaminolysis for survival. Senescent cells can avidly uptake glutamine and reduce glutamine availability for CSC.