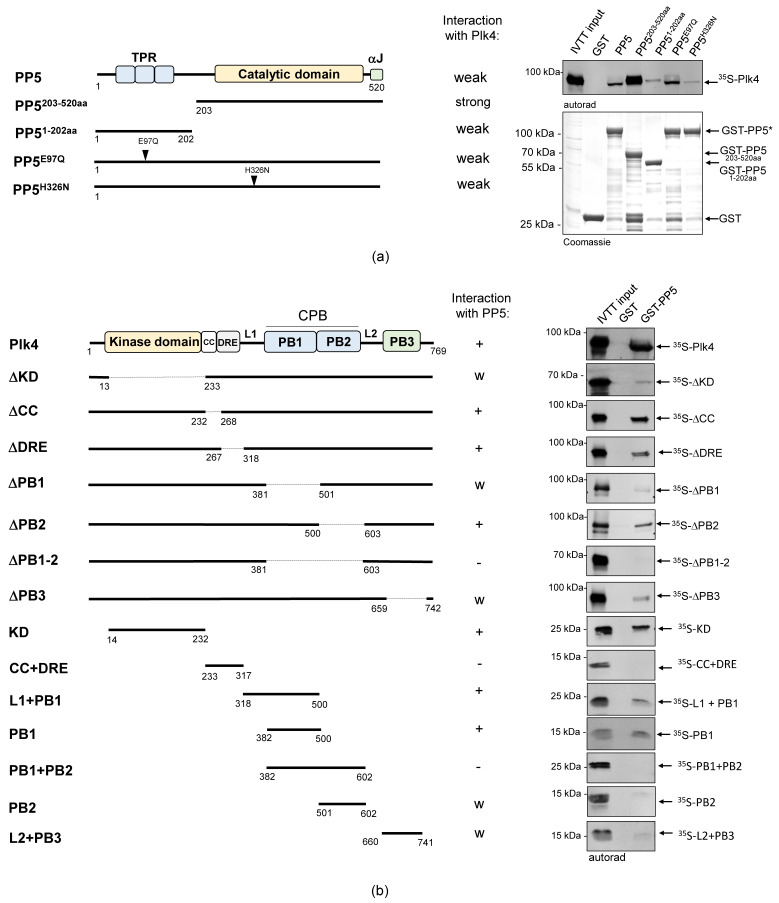
Figure 2.
The catalytic domain of PP5 interacts with the kinase domain and PB1 domain of Plk4. (a) GST-IVTT in vitro binding assay: autoradiogram (right panel) shows that in the absence of the TPR-domain (catalytic domain only), GST-PP5203−520aa binds more strongly to 35S−Plk4, in vitro. The wild type and hyperactive GST-PP5/PP5E97Q bind moderately, while the inactive GST-PP5H326N and the TPR domain-containing GST-PP51−202aa bind weakly to 35S-Plk4. The Coomassie brilliant blue-stained gel shows the loading of the bait proteins. The asterisk indicates the location of the wild type and point-mutant forms of GST-PP5. GST serves as the negative control. (b) Domain mapping of Plk4 was carried out with a GST-IVTT in vitro binding assay. Right panels (autoradiograms) show that GST-PP5 specifically binds to the kinase (KD) and PB1 domains of 35S-Plk4. GST serves as the negative control. “+” means strong binding; “−” means no binding; “w” means weak binding; “Δ” refers to the missing domain/motif of the full length protein (indicated by dashed lines in the diagram); numbers show the position of amino acid endpoints of the truncated proteins. Coomassie brilliant blue-stained gels (loading) are shown in Figure S2. Schematics on the left represent the domain/motif architecture and sizes of PP5 and Plk4 constructs used in this assay.