Figure 3.
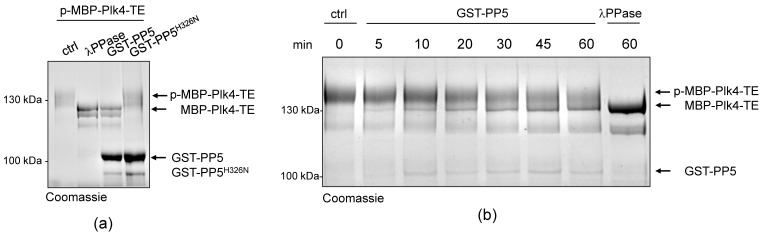
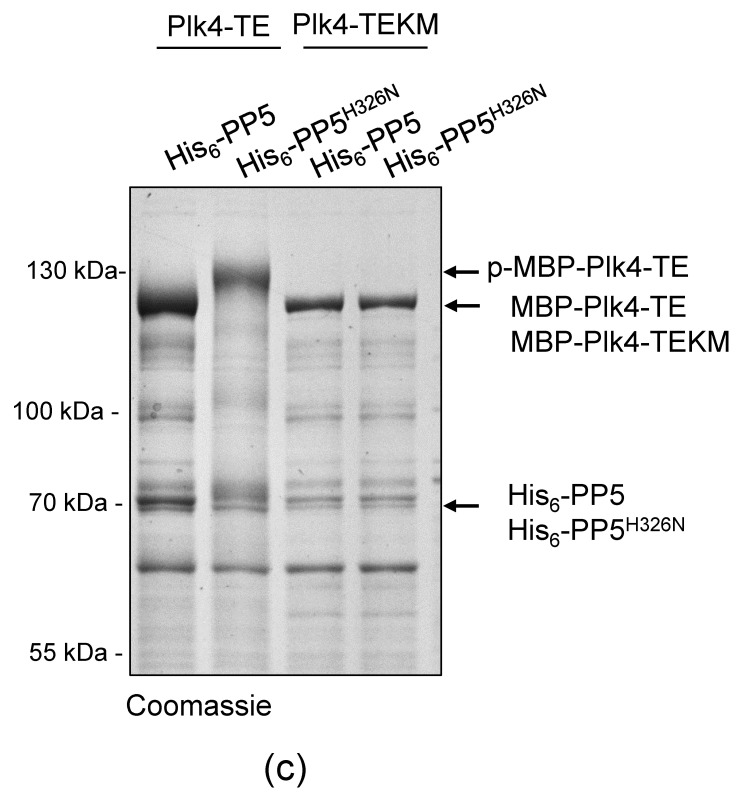
PP5 dephosphorylates Plk4, in vitro and in vivo. (a) In vitro dephosphorylation of p-MBP-Plk4-TE (“p-” refers to the self-phosphorylated, while “TE” refers to the T172E activating mutation in Plk4, respectively) was tested by gel-shift assay. The electrophoretic mobility of the phosphorylated forms is decreased, therefore they run higher, while the dephosphorylated species run lower in a long preparative SDS-PAGE. Purified recombinant p-MBP-Plk4-TE is efficiently dephosphorylated by the λ-phosphatase (λPPase) and GST-PP5, in vitro. GST-PP5H326N serves as the negative control; “ctrl” refers to non-treated purified kinase. (b) The GST-PP5-mediated dephosphorylation kinetics of p-MBP-Plk4-TE is presented by Coomassie brilliant blue-stained preparative SDS-PAGE. Incubation times (minutes) are indicated. λPPase serves as the positive control; “ctrl” refers to non-treated sample. (c) In vivo dephosphorylation of Plk4 in bacteria was tested by gel-shift assay. MBP-Plk4-TE or its kinase dead (TEKM) version were co-expressed with His6-PP5 or its inactive form, His6-PP5H326N, respectively, in bacteria. Crude cell lysates were analyzed by SDS-PAGE, which shows that MBP-Plk4-TE dephosphorylation requires the active His6-PP5, in vivo. MBP-Plk4-TEKM is not capable of self-phosphorylation. His6-PP5H326N is unable to dephosphorylate Plk4.