Figure 7.
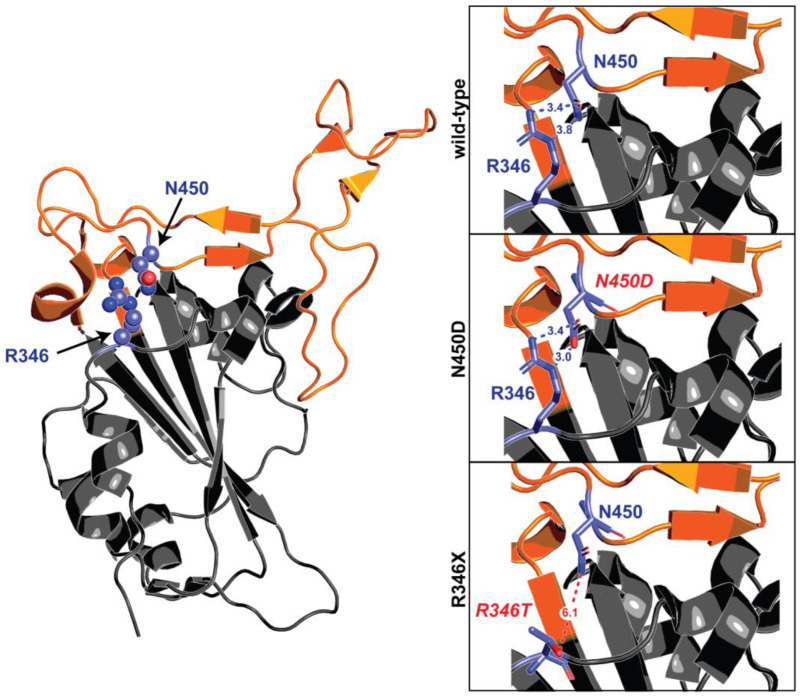
Mutually exclusive mutations at R346 and N450. The receptor binding domain of S is depicted in grey cartoon representation, with the receptor binding module (ACE2 interaction interface) highlighted in orange. Amino acids at the 346 and 450 positions are displayed as purple sticks. A zoomed-in view of the R346-N450 interaction in the ancestral domain, as well as the computationally modelled amino acid substitutions at those two positions, are portrayed in boxes to the right. In the wild-type sequence, the basic R346 sidechain interacts with the N450 residue through a pair of hydrogen bond interactions. N450D results in a similarly sized sidechain, but altered electrostatics. One hydrogen bond is maintained between the neutral oxygen of Asp and Nε of Arg, and a new salt bridge is formed between the anionic deprotonated oxygen of Asp and the cationic center of the guanidino group of Arg. In the case of R346X, any substitution except lysine would result in a side chain that is significantly shorter and non-cationic, thus dissolving the interactions between N450 or other common substitutions at that position.
