Table 1.
Overview of the four drugs and their respective targets and approval for use in the treatment of MTC.
| Drug | Targets | Dose | Approval for MTC | Structure |
|---|---|---|---|---|
| Cabozantinib [28,29] | MET, RET and VEGFR-2 | 140 mg/day | 2012 (FDA) 2013 (EMA |
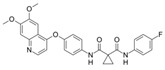
|
| Vandetanib [30,31] |
RET, VEGFR-2 and EGFR | 300 mg/day | 2012 (FDA) 2012 (EMA) |
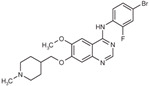
|
| Selpercatinib [32] | RET | 160 mg twice a day | 2020 (FDA) 2021 (EMA) |
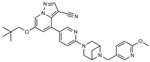
|
| Pralsetinib [26] | RET | 300mg/day | 2020 (FDA) NA (EMA) |
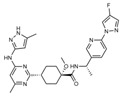
|
Only receptors inhibited by cabozantinib, which are being investigated in the EXAM study, are mentioned. FDA: Food and Drug Administration; EMA European Medicines Agency; NA: not applicable.
