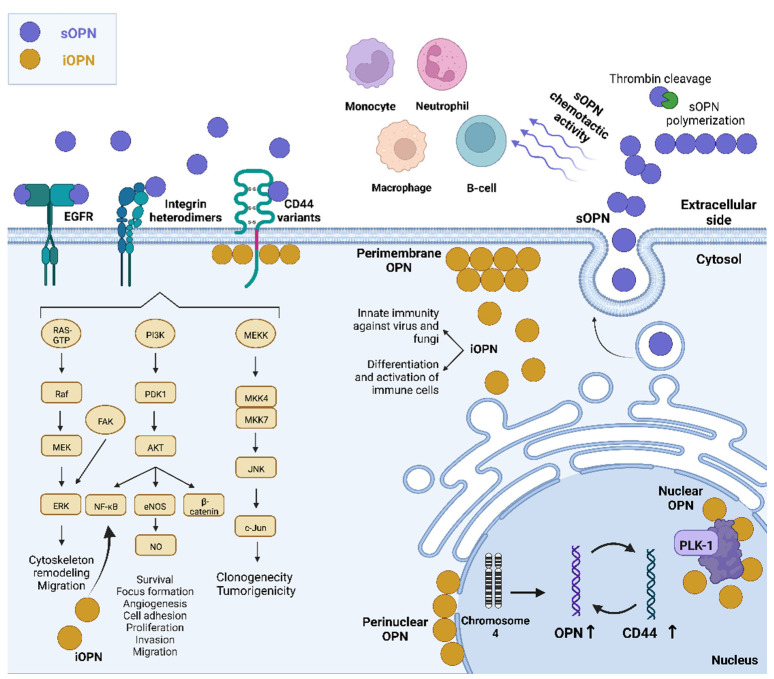
Figure 3.
Roles and mechanisms of osteopontin in cancer cells. Osteopontin is a highly expressed protein that acts through several mechanisms in cancer cells. Secreted OPN can regulate PI3K/AKT, MAPK, and JNK intracellular signaling pathways through interacting with its known membrane receptors, CD44 variants, integrins, and/or EGFR, resulting in the activation of a pro-tumorigenic phenotype, including increased survival, proliferation, clonogenicity and capacity of migration and invasion. Extracellular OPN has been extensively associated with the immune system once this protein mediates chemoattraction of cells, such as monocytes, neutrophils, and lymphocytes, as well as macrophage accumulation in diverse diseases, including in tumor microenvironments. This variety of extracellular roles is due to several modifications, such as post-translational mechanisms, polymerization, and thrombin cleavage. Intracellular OPN has been localized in the perimembranous and perinuclear regions regulating the expression of NF-kB, cytoskeleton remodeling through the CD44 receptor, and activation and differentiation of immune cells in responses against viruses and fungi. Besides cytoplasmic OPN, it has been observed a nuclear localization for this protein where it accumulates and is associated with the presence of Plk-1 and chromatin condensation. Once OPN has been associated with a poor prognosis and acquisition of pro-tumorigenic features, molecules aiming the regulation of OPN have been the focus of studies, such as genistein, which inhibits sOPN expression, leading to reduces colony formation rates, migration, and invasion. sOPN: secreted OPN. iOPN: intracellular OPN. Created with BioRender.com (accessed on 11 November 2022).