Abstract
Beneficial off-target effects of the Bacillus Calmette-Guérin (BCG) vaccination might offer general protection from respiratory tract infections. We conducted a systematic review and meta-analysis of published randomized controlled trials (RCTs) to ascertain BCG vaccination effectiveness against COVID-19. We looked up English RCTs from 1 January 2019 to 15 November 2022 in Embase, the Cochrane Library, and the Web of Science in this systematic review and meta-analysis. Nine RCTs, including 7963 participants, were included. The infection rate of COVID-19 was not decreased in people who were vaccinated with BCG (OR, 0.96; 95% CI, 0.82–1.13; I2 = 4%), and the BCG vaccination group did not have decreased COVID-19 related-hospitalization (OR, 0.66; 95% CI, 0.37–1.18; I2 = 42%), admission to the ICU (OR, 0.25; 95% CI, 0.05–1.18; I2 = 0%), and mortality (OR, 0.64; 95% CI, 0.17–2.44; I2 = 0%) compared with the control group. There is not sufficient evidence to support the use of BCG vaccination in the prevention of COVID-19 infection and severe COVID-19 and avoid overstating the role of BCG vaccination leading to its misuse.
Keywords: COVID-19, BCG, vaccine, trained immunity, SARS-CoV-2
1. Introduction
Since the end of 2019, a new virus known as severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has been sweeping the globe, triggering coronavirus disease 2019 (COVID-19).
Early in the 20th century, the Bacillus Calmette-Guerin (BCG) vaccine was created to prevent tuberculosis [1]. Furthermore, BCG has beneficial off-target (i.e., non-specific or heterologous) effects, which are becoming increasingly recognized. For example, BCG vaccination is related to lower all-cause mortality in babies and all-caused respiratory disease morbidity in the elderly, as well it can protect against human experimental models of yellow fever in healthy adults [2,3,4]. Due to the off-target effects of the BCG vaccine, it can alter immunologic set points via heterologous T-cell immunity or reprogramming of innate immune cells [3,5,6,7]. BCG vaccination might be more beneficial in conditions where such viral variations are abundant by enhancing antiviral host defense in an antigen-independent manner compared with the COVID-19 vaccine [8].
In current studies, it is controversial whether the BCG vaccine can be used against COVID-19 [9,10,11,12,13,14,15,16,17]. For example, Berg et al. found BCG vaccination could reduce mortality and morbidity rates by comparing the COVID-19 infection and death records from countries with a national BCG vaccination program to countries without [10]. However, the study of Arlehamn et al. did not support the assertion that BCG could reduce COVID-19 mortality when using updated mortality data [17]. Furthermore, Pépin et al. demonstrated that BCG vaccination did not reduce COVID-19 infection, hospitalization, or mortality rates [9].
To determine the efficacy of BCG vaccination against COVID-19, we conducted a systematic review and meta-analysis of randomized controlled trials.
2. Materials and Methods
2.1. Data Sources and Searches
The review protocol was registered in the PROSPERO International Prospective Register of Systematic Reviews (registration number: CRD42022339994) and conducted according to the preferred reporting items for systematic reviews and meta-analysis statements [18]. We searched PubMed, Embase, Cochrane Library, and Web of Science for relevant literature from 1 January 2019 to 15 November 2022. The search included the terms (“COVID-19” OR “SARS-CoV-2”) AND (“BCG Vaccine” OR “Mycobacterium Bovis”); the detailed search approach is outlined in the Supplementary Material (Supplementary Material: Appendix S1). In addition, manual backward searches of references from included studies were also conducted. The result was transferred to EndNote X9 for additional evaluation.
2.2. Selection of Studies
Inclusion criteria: (1) Population (P): Participants were adults (aged 18 years or over) who tested positive for COVID-19 by PCR or rapid antigen. There were no restrictions on gender, race, ethnicity, or geographical distribution; (2) Intervention (I): any strain BCG at any dosage (3) Control (C): placebo or no treatment; (4) Outcome (O): primary outcomes: The primary outcome is the incidence of COVID-19. The secondary outcomes were hospitalization, admission to the intensive care units (ICU)and mortality of COVID-19, and the risk of adverse events (AEs). All outcomes were followed for the longest follow-up periods for each one. Last, Randomized controlled trials (RCTs) in English published were included.
We excluded observational studies, research protocols, reviews, news, case reports, abstracts from conferences, unpublished publications accessible on preprint services, animal studies, in vivo experiments, animal research, and studies that did not test for COVID-19 infections.
2.3. Study Selection and Data Extraction
Two writers (JYW and QXL) independently read the titles and abstracts of the papers to find those that satisfied the inclusion criteria and extracted data. In the absence of unanimity, a third reviewer (JQH) was consulted. Information was extracted into an Excel spreadsheet. We extracted the following information: (1) first author; (2) year of publication; (3) participant characteristics; (4) intervention/exposure and control; (5) the type of COVID-19 diagnosis; and (6) data regarding BCG vaccination efficacy and risk of AEs as study outcomes were available.
2.4. Evaluation of the Risk of Bias and Quality
The risk of bias in RCTs was evaluated using RoB 2, a redesigned instrument for evaluating the risk of bias in randomized trials. RoB 2 was comprised of the following five domains: (1) randomization process, (2) deviations from intended interventions, (3) missing outcome data, (4) measurement of the outcome, and (5) selection of the reported result. Each of these domains is assigned a risk level that ranges from “High risk” to “Some concerns” to “Low risk” [19]. To assess the quality of evidence, GRADE (Grading of Recommendations Assessment, Development, and Evaluation) recommendations were used and were classified into “high,” “medium,” “low,” and “very low,” which were based on the risk of bias, consistency, directness, precision, and publication bias [20]. When one of the above criteria was not met, the assigned quality level was lowered.
The evaluations were carried out independently by two investigators (JYW and DYT). Disputes between the two investigators were resolved through discussion or by the third investigator (QXL).
2.5. Statistical Analysis
Continuous variables were reported as the mean and standard derivation (SD), while dichotomous variables were reported as the frequency and proportion. The effect size was summarized as odds ratios (ORs) with confidence intervals (CIs), which were displayed in forest plots. Statistical heterogeneity among studies was calculated by χ2-based Q test and I2 statistics, with I2 > 50% considered statistically significant. Values of 25%, 50%, and 75% were used as cutoff points for low, medium, and high inconsistency [21]. Pooled outcomes with 95% confidence intervals (95% CIs) were calculated using the random-effect model if I2 > 50%; otherwise, the fixed-effect model was used.
Sensitivity analyses were conducted by sequentially deleting studies to examine the robustness of the aggregated data. In addition, preplanned subgroup analyses were based on the strains in the vaccine. Each BCG strain belonged to a different subgroup that consisted of at least two studies.
Egger’s test and funnel plots were used to assess the presence of publication biases. Potential missing studies were imputed using the trim-and-fill method if publication bias was suspected (p < 0.05).
All statistical analyses were carried out using Stata (Version 16; StataCorp, College Station, TX, USA) and Review Manager, Version 5.4.1 (Cochrane Collaboration).
3. Results
3.1. Search Results and Study Characteristics
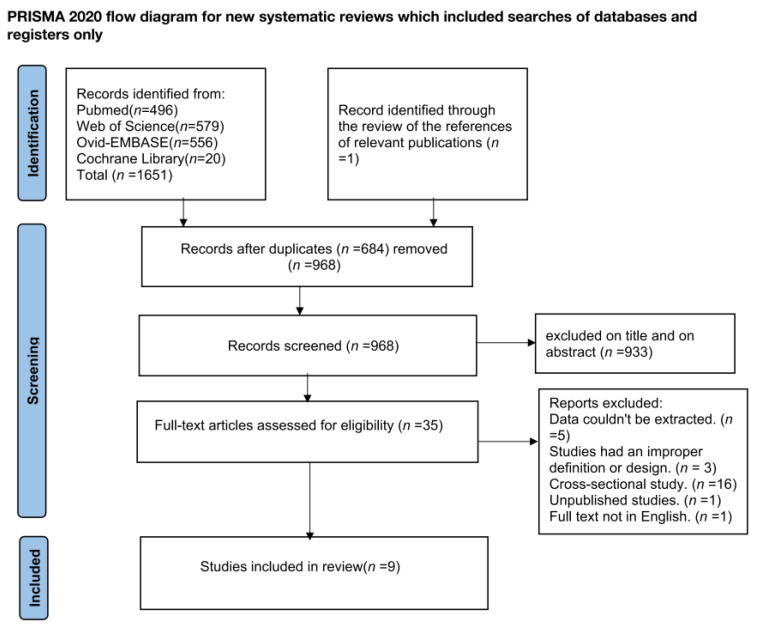
The search approach uncovered 1652 potentially relevant studies, of which 684 were omitted owing to duplication. Among the remaining 968 studies, 933 were excluded due to their titles or abstracts. Therefore, 35 studies were eligible for full-text review, and 26 studies were excluded for the following reasons: unavailable data (n = 5), improper definition and design (n = 3), observational studies (n = 16), unpublished study (n = 1), and full-text not in English (n = 1). Eventually, the current study consisted of a total of nine randomized controlled trials that enrolled 7963 participants and were published in 2022 [22,23,24,25,26,27,28,29,30] (Figure 1).
Figure 1.
The detailed flow chart of the Literature search.
In all of the studies that were included, the experimental and control groups were well-balanced in terms of baseline demographics, such as age and gender ratio [22,23,24,25,26,27,28,29,30]. Seven of the nine included studies reported that the precise BCG vaccination history [22,23,24,25,26,27,29] and comorbidities [23,24,25,26,27,28,29] were balanced between the two groups, with no statistically significant difference. However, two lacked information on the study participants’ precise BCG vaccination history [28,30] and comorbidities [22,30]. All participants were given intradermal BCG.
Among the nine RCTs, five were multicenter studies (Poland [22], Greece [28], the Netherlands [23,26], and Germany [30]), and four were single-center studies (Brazil [24], South Africa [29], the United States [25] and India [27]).
Three studies included data on older adults [26,28,30], one on patients with type 1 diabetes [25], and the remaining four studies enrolled healthcare workers [22,23,24,29]. For the diagnosis of COVID-19, seven studies solely relied on a positive PCR test [22,23,26,27,28,29,30], one study depended on both a positive PCR test and symptoms [25], and another study relied on either the positive PCR test or rapid antigen test [24]. Moreover, the sample size ranged from 131 to 2015, and the follow-up time spanned from 3 to 15 months. The detailed information is shown in Table 1.
Table 1.
Study characteristics and participant demography.
| Study | Year Published | Study Design | Participants Characteristics | Group | Timing of Follow-Up | Diagnosis of COVID-19 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (Mean, Year) | Mean Age (y) | Total Number (N) | Male (%) | Intervention (BCG Stain and Dosage) | Control | |||||
| Blossey [30] | 2022 | RCT multicenter | Older Adults | 67.3 | 2025 | 52.9 | BCG vaccination (VPM1002) (a genetically modified BCG) (2–8 × 105 colony forming units) | placebo | 240 days | positive PCR |
| Sinha [27] | 2022 | RCT multicenter | Adults’ underlying medical conditions | 43 | 495 | 52.1 | 0.1 mL BCG Moscow | Placebo | 9 months | positive PCR |
| Tsilika [28] | 2022 | RCT Single center | Adults at risk | 69 | 301 | 67.8 | 0.1 mL BCG Moscow | Placebo | 6 months | positive PCR |
| Faustman [25] | 2022 | RCT Single center | Patients with type 1 diabetes | 43.8 | 144 | 58.3 | Multiple Tokyo 172 strain | Placebo | 15 months | positive PCR and symptoms |
| Upton [29] | 2022 | RCT multicenter | Healthcare workers | 39 | 1000 | 29.6 | 0.1 mL Danish strain 1331 | Placebo | 52 weeks | positive PCR |
| Doesschate [23] | 2022 | RCT multicenter | Healthcare workers | 42 | 1511 | 25.7 | 0.1 mL Danish strain 1331 | Placebo | 26 weeks | positive PCR |
| Dos Anjos [24] | 2022 | RCT Single center | Healthcare workers | 43 | 131 | 23.7 | 0.1 mL BCG Moscow | Unvaccinated | 180 days | Positive PCR or rapid antigen test |
| Moorlag [26] | 2022 | RCT Single center | Older Adults | 67 | 2014 | 52.5 | 0.1 mL Danish strain 1331 | Placebo | 12 months | positive PCR |
| Czajka [22] | 2022 | RCT multicenter | Healthcare workers | 45 | 342 | 19.3 | BCG vaccination in TST (-) participants Moreau Strain | Placebo in TST (-) participants | 3 months | Positive PCR |
BCG: Bacille Calmette-Guerin; TST: Tuberculin test results; RCT: a randomized controlled trial; PCR: polymerase chain reaction; COVID-19: Coronavirus disease 2019.
3.2. Primary Outcomes
The Rate of Infection of COVID-19
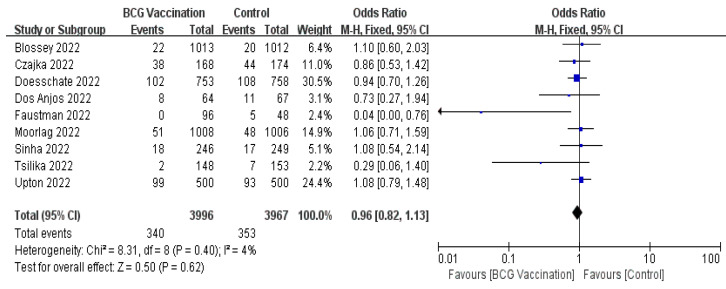
A total of nine RCTs [22,23,24,25,26,27,28,29,30], including 7963 participants, evaluated BCG vaccination effectiveness against COVID-19. The results showed that the incidence of COVID-19 infection was not significantly decreased in people who were vaccinated with BCG using the fixed-effect model (OR, 0.96; 95% CI, 0.82–1.13; Figure 2). There was low heterogeneity among all included studies of COVID-19 (I2 = 4%, p = 0.4; Figure 2).
Figure 2.
Forrest plot of the odds ratio of the incidence of COVID-19 between the BCG vaccination group and the control group.
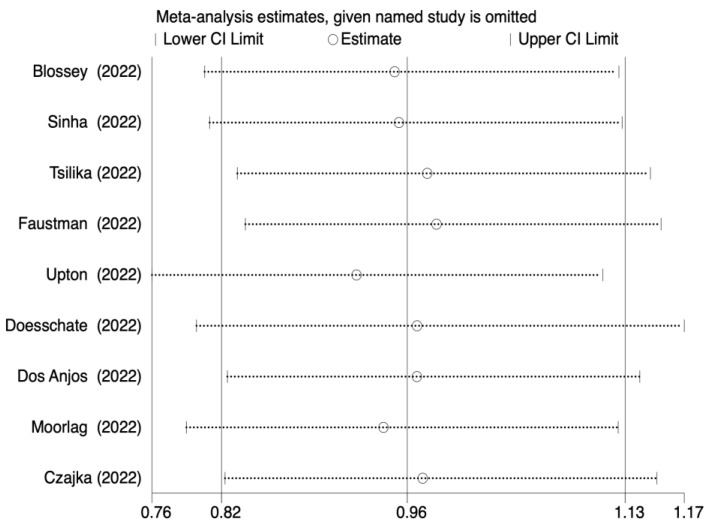
The leave-one-out sensitivity analysis also indicated that individual research studies did not affect the pooled incidence rate (Figure 3). An analysis of the subgroup that had been preplanned based on the BCG strain was carried out. The results also did not change in the BCG Moscow group (OR, 0.81; 95% CI, 0.48–1.35; I2 = 15%) and Danish strain 1331 group (OR, 1.02; 95% CI, 0.84–1.23; I2 = 0%). There was no intergroup heterogeneity in the two groups (I2 = 15%, p = 0.31; I2 = 0%, p = 0.80, respectively) (Supplementary Material: Appendix S2 Figure SA1).
Figure 3.
Sensitivity analysis result of the association between BCG vaccination and incidence of COVID-19.
3.3. Secondary Outcomes
3.3.1. The COVID-19-Related Hospitalization
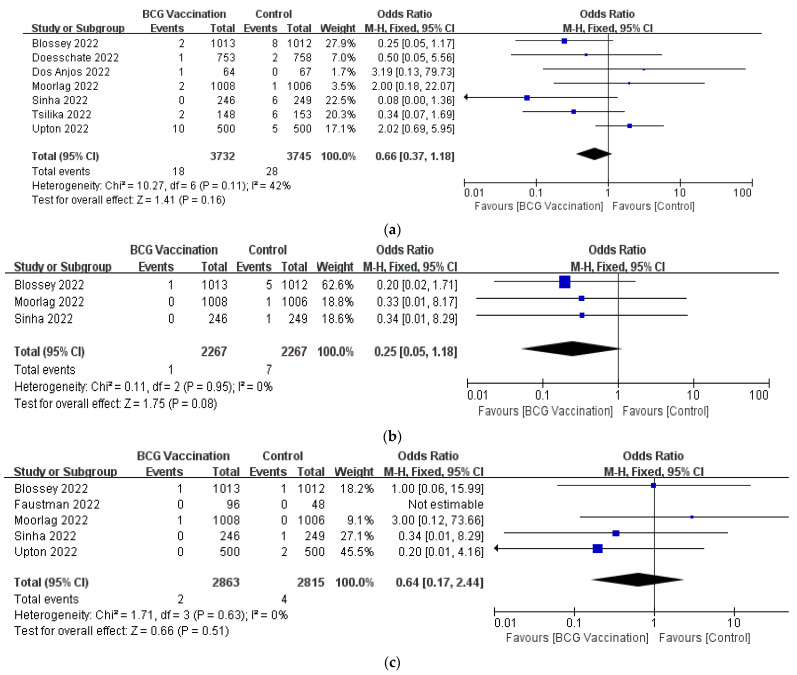
All seven studies reported COVID-19 hospitalizations [23,24,26,27,28,29,30]. According to the fixed-effect model, there was no statistically significant difference between the BCG vaccination group and the control group (OR, 0.66; 95% CI, 0.37–1.18; I2 = 42%) (Figure 4a).
Figure 4.
Forrest plot of the odds ratio of the (a) hospitalization; (b) admission to the ICU; (c) mortality of COVID-19 between the BCG vaccination group and the control group.
3.3.2. The COVID-19-Related Admission to the ICU
There was no significant difference observed in COVID-19-related admission to the ICU between participants vaccinated or not vaccinated with BCG of three RCTs (OR, 0.25; 95% CI, 0.05–1.18; I2 = 0%) (Figure 4b) [26,27,30].
3.3.3. The COVID-19-Related Mortalitys
A fixed effect meta-analysis of five trials showed no significant difference between the BCG vaccination and control group in terms of COVID-19-related mortality (OR, 0.64; 95% CI, 0.17–2.24; I2 = 0%) (Figure 4c) [25,26,27,29,30].
3.3.4. The Safety of BCG Vaccination
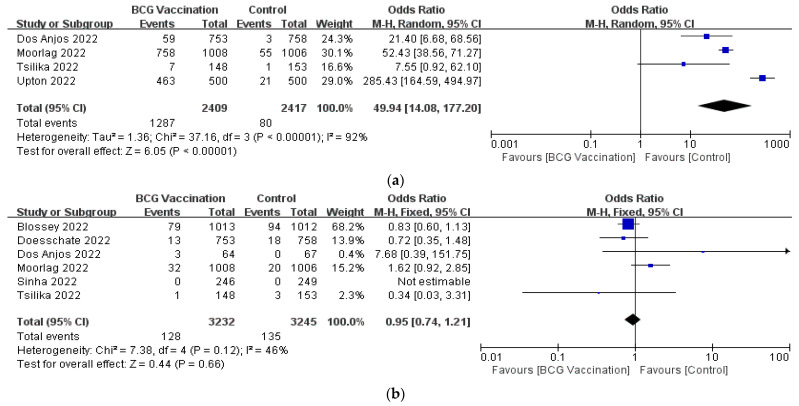
In terms of specific AEs, we found local injection reactions were more common in the BCG group compared to the control group in four RCTs (OR, 49.94; 95% CI, 14.08–177.2) and there was high heterogeneity between the studies (p < 0.00001; I2 = 92%) [24,26,28,29] (Figure 5a). The subgroup analyses according to the BCG strain for both the BCG Moscow (OR, 17.88; 95% CI, 6.49–49.25; I2 = 0%) and BCG Danish 1331 groups (OR, 76.23; 95% CI, 58.42–99.48; I2 = 96%) showed similar results, and there was still substantial heterogeneity in the Danish strain 1331 group (Appendix S2 Figure SA2). Six RCTs reported serious AEs, but there were no discernible differences between them (OR, 0.95; 95%CI, 0.74–1.21; I2 = 46%) [2,23,24,26,27,28] (Figure 5b).
Figure 5.
Forrest plot of the odds ratio of the (a) local injection response; (b) serious AEs between the BCG vaccination group and the control group.
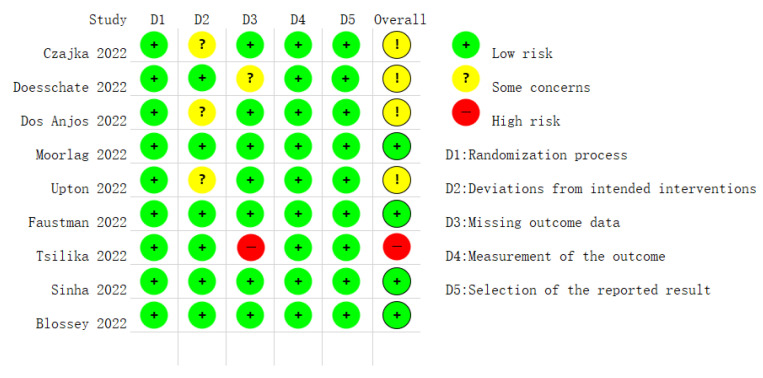
3.4. Risk of Bias and Publication Bias
For nine RCTs, four were judged to have low bias concerns [26,27,29,30], while four were judged to have some concerns of bias regarding “deviations from intended interventions” and “missing outcome data” [22,23,24,29]. In addition, one study had a high-risk bias for missing outcome data [28]. The comprehensive evaluation of bias risk is given in Figure 6.
Figure 6.
Quality assessment of the risk of bias in the studies.
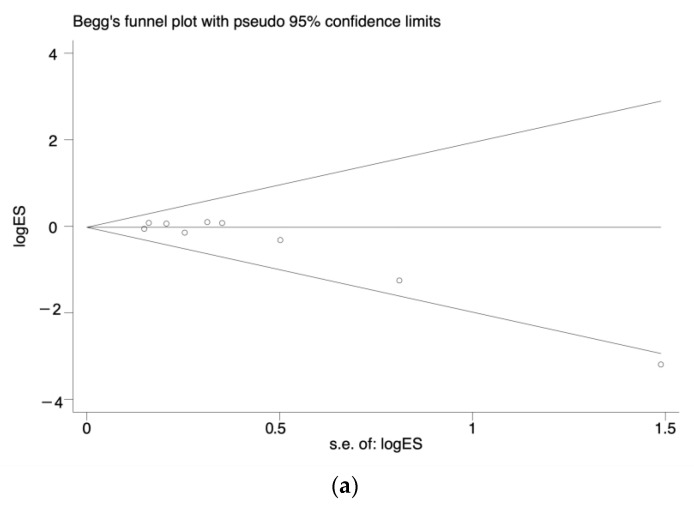
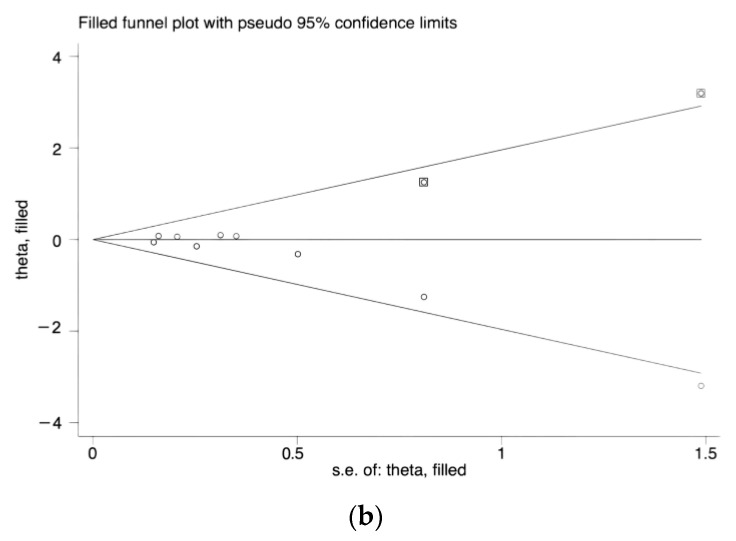
For the primary outcome, the asymmetry shown by visual inspection of the funnel plot (Figure 7a) was statistically significant, and Egger’s test was similar (p = 0.017). To further verify its influence on results, trim-and-fill adjustment was performed. Adding two studies had no significant impact on the adjusted results of our primary outcome (fixed effects model, OR, 1.00; 95% CI, 0.85–1.17) or (random effects model, OR, 1.00; 95% CI, 0.79–1.26) (Figure 7b). For secondary outcomes, which included COVID-19-related hospitalization, COVID-19-related mortality, and serious AEs, there was no evidence of publishing biases. The funnel plots were displayed in the Supplementary Material: Appendix S2 Figure SA3. No publication bias tests were performed for the COVID-19-related ICU and local injection responses since there were not enough trials.
Figure 7.
Publication bias analysis of the infection of COVID-19 between the BCG vaccination group and control: (a) The Funnel plot. (b) Trim and fill analysis.
3.5. Grade Evaluation
Table 2 displayed GRADE assessments of the quality of evidence for all outcomes. The primary outcome was evaluated as ‘moderate’ due to publication bias.
Table 2.
GRADE assessment on the quality of the evidence.
| Outcomes | No of Participants (Studies) |
Quality of the Evidence (GRADE) |
Relative Effect (95% CI) |
Anticipated Absolute Effects | |
|---|---|---|---|---|---|
| Risk with Control | Risk Difference with BCG Vaccination (95% CI) | ||||
| The rate of infection of COVID-19 | 7963 (9 studies) |
⊕⊕⊕⊝ MODERATE 1 due to publication bias |
OR 0.96 (0.82 to 1.13) |
Study population | |
| 89 per 1000 |
3 fewer per 1000 (from 15 fewer to 10 more) |
||||
| Moderate | |||||
| 104 per 1000 |
4 fewer per 1000 (from 17 fewer to 12 more) |
||||
| The rate of COVID-19-related hospitalization | 7477 (7 studies) |
⊕⊕⊕⊕ HIGH |
OR 0.66 (0.37 to 1.18) |
Study population | |
| 7 per 1000 |
3 fewer per 1000 (from 5 fewer to 1 more) |
||||
| Moderate | |||||
| 8 per 1000 |
3 fewer per 1000 (from 5 fewer to 1 more) |
||||
| The rate of COVID-19-related admission to the ICU | 4534 (3 studies) |
⊕⊕⊝⊝ LOW 2 due to imprecision |
OR 0.25 (0.05 to 1.18) |
Study population | |
| 3 per 1000 |
2 fewer per 1000 (from 3 fewer to 1 more) |
||||
| Moderate | |||||
| 4 per 1000 |
3 fewer per 1000 (from 4 fewer to 1 more) |
||||
| The rate of COVID-19-related mortality | 5678 (5 studies) |
⊕⊕⊝⊝ LOW 3 due to imprecision |
OR 0.64 (0.17 to 2.44) |
Study population | |
| 1 per 1000 |
1 fewer per 1000 (from 1 fewer to 2 more) |
||||
| Moderate | |||||
| 1 per 1000 |
0 fewer per 1000 (from 1 fewer to 1 more) |
||||
| The rate of local injection response. | 4826 (4 studies) |
⊕⊕⊝⊝ LOW 4 due to inconsistency |
OR 49.94 (14.08 to 177.2) |
Study population | |
| 33 per 1000 |
598 more per 1000 (from 292 more to 825 more) |
||||
| Moderate | |||||
| 24 per 1000 |
527 more per 1000 (from 233 more to 789 more) |
||||
| The rate of local serious AEs. | 6477 (6 studies) |
⊕⊕⊕⊕ HIGH |
OR 0.95 (0.74 to 1.21) |
Study population | |
| 42 per 1000 |
2 fewer per 1000 (from 10 fewer to 8 more) |
||||
| Moderate | |||||
| 20 per 1000 |
1 fewer per 1000 (from 5 fewer to 4 more) |
||||
CI: Confidence interval; OR: Odds ratio; ICU: Intensive care units; AEs: Adverse events; COVID-19: Coronavirus disease 2019. GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. 1 Funnel plot asymmetry and/or significant Egger’s test (p = 0.017). 2 The number of events is small. 3 The number of events is small. 4 The heterogeneity is high (I2 = 92%).
4. Discussion
In this study, we assessed the effectiveness of BCG vaccination against COVID-19. This meta-analysis demonstrated that BCG vaccination did not significantly provide prevention for COVID-19. The following data provided support for this conclusion. First, we discovered that, compared to the control group, those who received the BCG vaccination had no significantly lower incidence of COVID-19 infection (OR, 0.97; 95% CI, 0.84–1.11) with low heterogeneity (I2 = 4%). Second, the result did not change in the leave-one-out sensitivity analysis. Furthermore, the BCG vaccine was first introduced in 1921. During their passage, BCG strains accumulated genomic alterations leading to the emergence of several substrains. It has been discovered that the immune response amplitude varies according to the strain [31,32]. We analyzed subgroup analyses based on BCG strain; the results were similar.
In addition, our study also discovered BCG vaccination could not prevent severe COVID-19 in terms of COVID-19-related hospitalization (OR, 0.66; 95% CI, 0.37–1.18; I2 = 42%), admission to the ICU (OR, 0.25; 95% CI, 0.05–1.18; I2 = 0%), and mortality (OR, 0.64; 95% CI, 0.17–2.44; I2 = 0%). In addition, we found the BCG vaccination group experienced more local injection response (OR, 49.94; 95% CI, 14.08–177.2; I2 = 92%) compared with the control group, but the serious AEs were similar (OR, 0.95; 95%CI, 0.74–1.21; I2 = 46%). By subgroup analysis, we found the high heterogeneity of local injection response did not originate from the type of BCG strain and suspected it might be related to the lack of a precise definition of a local injection reaction. In general, BCG vaccination is relatively safe.
The same result was concluded when Roborovski hamsters were subcutaneously vaccinated with BCG. Extensive damage to the pulmonary vasculature and significant levels of SARS-CoV-2 RNA were detected in the bone marrow of infected mice. Because off-target effects greatly relied on hematopoietic progenitor, which grew and developed in the bone marrow, SARS-CoV-2 was speculated to prevent off-target effects by damaging pulmonary vasculature and disseminating, which was a unique feature compared with other respiratory infections, such as influenza A virus (IAV) [16]. In conclusion, they speculated that due to the tissue tropism of COVID-19, BCG vaccination could not provide protection.
Similarly, Hilligan et al. demonstrated that BCG vaccination subcutaneously did not prevent SARS-CoV-2 infection nor severe COVID-19 in K18-hACE2 mice [33]. However, they found that intravenous injection of BCG can protect against lethal infection by reducing SCV2-induced tissue pathology, inflammatory cell recruitment, and excessive cytokine inflammatory responses [33,34]. In our study, BCG vaccination did not prevent severe COVID-19, possibly because intradermal BCG vaccination for humans is generally recommended except for treating bladder cancer. In contrast to intravenous vaccination in mice, human intradermal BCG vaccination is similar to subcutaneous vaccination in mice.
Participants in all RCTs were vaccinated with COVID-19–specific vaccines at a later stage of the trial, and no differences were observed between the two groups. The current study showed BCG vaccination could enhance the efficacy of the SARS-CoV-2 vaccine utilizing boosting antibody and memory T-cell responses [35], but no differences in the rates of infection and hospitalization or mortality were found in the two groups. It has to be further investigated whether the strengthening of antibody and memory T-cell responses is powerful enough to prevent infection or reduce severe infection, and our study does not support BCG as a booster for the COVID-19 vaccine.
As opposed to what we found, a recent meta-analysis showed that BCG vaccination could protect against SARS-CoV-2 infection (OR, 0.61; 95% CI, 0.39–0.95; I2 = 31%) [36], perhaps because it included only (not merely) observational studies.
The study’s limitations are as follows:(1) The number of RCTs we included is small. Although more than forty clinical trials of BCG vaccination against COVID-19 have been registered, less than ten reports of these trials have been published [37]. For example, several clinical trials about the BCG vaccine to prevent COVID-19 for Health Care Workers are still ongoing in phase III trials [38,39,40,41]. Attention to the results of clinical trials in the coming years is needed to draw more accurate conclusions. (2) Only published studies were included because it is challenging to verify data in unpublished studies, especially when they have not undergone the rigorous peer review process. Although we discovered publication bias for the primary result, the trim-and-fill modification was conducted to guarantee the reliability of the results. (3) There was no information on the status of the measles vaccine (MV), oral polio vaccine (OPV), and measles-mumps-rubella (MMR), which may play the same role as BCG in “trained immunity” [42,43].
Regarding the strengths, in the meta-analysis, only RCTs were included, and the low heterogeneity ensured the credibility of the evidence. In addition, the sensitivity analysis performed on our primary outcome (the rate of infection) did not show noteworthy differences when deleting individual studies. Lastly, our findings support the 2020 WHO (World Health Organization) recommendation against using BCG vaccination to prevent COVID-19, which has important implications for avoiding unnecessary vaccination costs and a shortage of BCG vaccines [44].
5. Conclusions
Current findings do not support the assumption that BCG vaccination can protect against COVID-19 in terms of infection rate, admission to COVID-19-related hospitalization, admission to the ICU, and mortality. However, the number of RCTs we included is small, and the results of ongoing RCTs are important to validate this finding.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12031154/s1, Appendix S1: Search strategy; Appendix S2 Figure SA1: Subgroup analysis for the incidence of COVID-19 between the BCG vaccination group and the control group based on BCG strain; Figure SA2: Subgroup analysis for the incidence of local injection response between the BCG vaccination group and the control group based on BCG strain; Figure SA3: Publication bias analysis of the (a) COVID-19-related hospitalization; (b) COVID-19-related mortality; (c) serious AEs.
Author Contributions
The study was created and designed by J.W.; J.W. and Q.L. conducted the literature review and data extraction; The statistical analysis, figures, and appendix were encoded by D.T., J.-Q.H. and J.W. evaluated the data and drafted the initial version of the paper. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Because the data for this research were collected from published articles, ethical review and approval was waived.
Data Availability Statement
The datasets used and/or analyzed during this investigation are available upon reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Natural Science Foundation of China (Grant No. 81870015).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Lobo N., Brooks N.A., Zlotta A.R., Cirillo J.D., Boorjian S., Black P.C., Meeks J.J., Bivalacqua T.J., Gontero P., Steinberg G.D., et al. 100 years of Bacillus Calmette-Guérin immunotherapy: From cattle to COVID-19. Nat. Rev. Urol. 2021;18:611–622. doi: 10.1038/s41585-021-00481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biering-Sørensen S., Aaby P., Lund N., Monteiro I., Jensen K.J., Eriksen H.B., Schaltz-Buchholzer F., Jørgensen A.S.P., Rodrigues A., Fisker A.B., et al. Early BCG-Denmark and Neonatal Mortality Among Infants Weighing < 2500 g: A Randomized Controlled Trial. Clin. Infect. Dis. 2017;65:1183–1190. doi: 10.1093/cid/cix525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arts R.J.W., Moorlag S., Novakovic B., Li Y., Wang S.Y., Oosting M., Kumar V., Xavier R.J., Wijmenga C., Joosten L.A.B., et al. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell. Host Microbe. 2018;23:89–100.e5. doi: 10.1016/j.chom.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Giamarellos-Bourboulis E.J., Tsilika M., Moorlag S., Antonakos N., Kotsaki A., Domínguez-Andrés J., Kyriazopoulou E., Gkavogianni T., Adami M.E., Damoraki G., et al. Activate: Randomized Clinical Trial of BCG Vaccination against Infection in the Elderly. Cell. 2020;183:315–323.e9. doi: 10.1016/j.cell.2020.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Netea M.G., Giamarellos-Bourboulis E.J., Domínguez-Andrés J., Curtis N., van Crevel R., van de Veerdonk F.L., Bonten M. Trained Immunity: A Tool for Reducing Susceptibility to and the Severity of SARS-CoV-2 Infection. Cell. 2020;181:969–977. doi: 10.1016/j.cell.2020.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cirovic B., de Bree L.C.J., Groh L., Blok B.A., Chan J., van der Velden W., Bremmers M.E.J., van Crevel R., Händler K., Picelli S., et al. BCG Vaccination in Humans Elicits Trained Immunity via the Hematopoietic Progenitor Compartment. Cell Host Microbe. 2020;28:322–334.e5. doi: 10.1016/j.chom.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh A.K., Netea M.G., Bishai W.R. BCG turns 100: Its nontraditional uses against viruses, cancer, and immunologic diseases. J. Clin. Investig. 2021;131:e148291. doi: 10.1172/JCI148291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng B., Gao L., Zhou Q., Yu K., Sun F. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern: A systematic review and meta-analysis. BMC Med. 2022;20:200. doi: 10.1186/s12916-022-02397-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pépin J., Labbé A.C., Carignan A., Parent M.E., Yu J., Grenier C., Beauchemin S., De Wals P., Valiquette L., Rousseau M.C. Does BCG provide long-term protection against SARS-CoV-2 infection? A case-control study in Quebec, Canada. Vaccine. 2021;39:7300–7307. doi: 10.1016/j.vaccine.2021.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berg M.K., Yu Q., Salvador C.E., Melani I., Kitayama S. Mandated Bacillus Calmette-Guérin (BCG) vaccination predicts flattened curves for the spread of COVID-19. Sci. Adv. 2020;6:eabc1463. doi: 10.1126/sciadv.abc1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J., Zhan L., Qin C. The double-sided effects of Mycobacterium Bovis bacillus Calmette-Guérin vaccine. NPJ Vaccines. 2021;6:14. doi: 10.1038/s41541-020-00278-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redelman-Sidi G. Could BCG be used to protect against COVID-19? Nat. Rev. Urol. 2020;17:316–317. doi: 10.1038/s41585-020-0325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Neill L.A.J., Netea M.G. BCG-induced trained immunity: Can it offer protection against COVID-19? Nat. Rev. Immunol. 2020;20:335–337. doi: 10.1038/s41577-020-0337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mariita R.M., Musila J.M. A study on the relationship between Bacillus CalmetteGurin (BCG) vaccination and COVID-19 prevalence: Do other confounders warrant investigation? J. Public Health Epidemiol. 2020;12:142–150. doi: 10.5897/JPHE2020.1230. [DOI] [Google Scholar]
- 15.Messina N.L., Germano S., McElroy R., Rudraraju R., Bonnici R., Pittet L.F., Neeland M.R., Nicholson S., Subbarao K., Curtis N. Off-target effects of bacillus Calmette-Guérin vaccination on immune responses to SARS-CoV-2: Implications for protection against severe COVID-19. Clin. Transl. Immunol. 2022;11:e1387. doi: 10.1002/cti2.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufmann E., Khan N., Tran K.A., Ulndreaj A., Pernet E., Fontes G., Lupien A., Desmeules P., McIntosh F., Abow A., et al. BCG vaccination provides protection against IAV but not SARS-CoV-2. Cell Rep. 2022;38:110502. doi: 10.1016/j.celrep.2022.110502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindestam Arlehamn C.S., Sette A., Peters B. Lack of evidence for BCG vaccine protection from severe COVID-19. Proc. Natl. Acad. Sci. USA. 2020;117:25203–25204. doi: 10.1073/pnas.2016733117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.Y., Corbett M.S., Eldridge S.M., et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 20.Balshem H., Helfand M., Schünemann H.J., Oxman A.D., Kunz R., Brozek J., Vist G.E., Falck-Ytter Y., Meerpohl J., Norris S., et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Czajka H., Zapolnik P., Krzych Ł., Kmiecik W., Stopyra L., Nowakowska A., Jackowska T., Darmochwał-Kolarz D., Szymański H., Radziewicz-Winnicki I., et al. A Multi-Center, Randomised, Double-Blind, Placebo-Controlled Phase III Clinical Trial Evaluating the Impact of BCG Re-Vaccination on the Incidence and Severity of SARS-CoV-2 Infections among Symptomatic Healthcare Professionals during the COVID-19 Pandemic in Poland-First Results. Vaccines. 2022;10:314. doi: 10.3390/vaccines10020314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doesschate T.T., van der Vaart T.W., Debisarun P.A., Taks E., Moorlag S., Paternotte N., Boersma W.G., Kuiper V.P., Roukens A.H.E., Rijnders B.J.A., et al. BCG vaccine to reduce healthcare worker absenteeism in COVID-19 pandemic, a randomized controlled trial. Clin. Microbiol. Infect. 2022;28:1278–1285. doi: 10.1016/j.cmi.2022.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dos Anjos L.R.B., da Costa A.C., Cardoso A., Guimarães R.A., Rodrigues R.L., Ribeiro K.M., Borges K.C.M., Carvalho A.C.O., Dias C.I.S., Rezende A.O., et al. Efficacy and Safety of BCG Revaccination With M. bovis BCG Moscow to Prevent COVID-19 Infection in Health Care Workers: A Randomized Phase II Clinical Trial. Front. Immunol. 2022;13:841868. doi: 10.3389/fimmu.2022.841868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faustman D.L., Lee A., Hostetter E.R., Aristarkhova A., Ng N.C., Shpilsky G.F., Tran L., Wolfe G., Takahashi H., Dias H.F., et al. Multiple BCG vaccinations for the prevention of COVID-19 and other infectious diseases in type 1 diabetes. Cell Rep. Med. 2022;3:100728. doi: 10.1016/j.xcrm.2022.100728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moorlag S.J.C.F.M., Taks E., Ten Doesschate T., van der Vaart T.W., Janssen A.B., Müller L., Ostermann P., Dijkstra H., Lemmers H., Simonetti E., et al. Efficacy of Bacillus Calmette-Guérin vaccination against respiratory tract infections in the elderly during the COVID-19 pandemic. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022 doi: 10.1093/cid/ciac182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinha S., Ajayababu A., Thukral H., Gupta S., Guha S.K., Basu A., Gupta G., Thakur P., Lingaiah R., Das B.K., et al. Efficacy of Bacillus Calmette-Guérin (BCG) Vaccination in Reducing the Incidence and Severity of COVID-19 in High-Risk Population (BRIC): A Phase III, Multi-centre, Quadruple-Blind Randomised Control Trial. Infect. Dis. Ther. 2022;11:2205–2217. doi: 10.1007/s40121-022-00703-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsilika M., Taks E., Dolianitis K., Kotsaki A., Leventogiannis K., Damoulari C., Kostoula M., Paneta M., Adamis G., Papanikolaou I., et al. ACTIVATE-2: A Double-Blind Randomized Trial of BCG Vaccination Against COVID-19 in Individuals at Risk. Front. Immunol. 2022;13:873067. doi: 10.3389/fimmu.2022.873067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Upton C.M., van Wijk R.C., Mockeliunas L., Simonsson U.S.H., McHarry K., van den Hoogen G., Muller C., von Delft A., van der Westhuizen H.M., van Crevel R., et al. Safety and efficacy of BCG re-vaccination in relation to COVID-19 morbidity in healthcare workers: A double-blind, randomised, controlled, phase 3 trial. EClinicalMedicine. 2022;48:101414. doi: 10.1016/j.eclinm.2022.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blossey A.M., Brückner S., May M., Parzmair G.P., Sharma H., Shaligram U., Grode L., Kaufmann S.H.E., Netea M.G., Schindler C. VPM1002 as Prophylaxis Against Severe Respiratory Tract Infections Including COVID-19 in the Elderly: A phase III randomised, double-blind, placebo-controlled, multicenter clinical study. Clin. Infect. Dis. 2022 doi: 10.1093/cid/ciac881. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L., Ru H.W., Chen F.Z., Jin C.Y., Sun R.F., Fan X.Y., Guo M., Mai J.T., Xu W.X., Lin Q.X., et al. Variable Virulence and Efficacy of BCG Vaccine Strains in Mice and Correlation With Genome Polymorphisms. Mol. Ther. 2016;24:398–405. doi: 10.1038/mt.2015.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ritz N., Hanekom W.A., Robins-Browne R., Britton W.J., Curtis N. Influence of BCG vaccine strain on the immune response and protection against tuberculosis. FEMS Microbiol. Rev. 2008;32:821–841. doi: 10.1111/j.1574-6976.2008.00118.x. [DOI] [PubMed] [Google Scholar]
- 33.Hilligan K.L., Namasivayam S., Clancy C.S., O’Mard D., Oland S.D., Robertson S.J., Baker P.J., Castro E., Garza N.L., Lafont B.A.P., et al. Intravenous administration of BCG protects mice against lethal SARS-CoV-2 challenge. J. Exp. Med. 2022;219:e20211862. doi: 10.1084/jem.20211862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang B.Z., Shuai H., Gong H.R., Hu J.C., Yan B., Yuen T.T., Hu Y.F., Yoon C., Wang X.L., Hou Y., et al. Bacillus Calmette-Guérin-induced trained immunity protects against SARS-CoV-2 challenge in K18-hACE2 mice. JCI Insight. 2022;7:e157393. doi: 10.1172/jci.insight.157393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rakshit S., Adiga V., Ahmed A., Parthiban C., Chetan Kumar N., Dwarkanath P., Shivalingaiah S., Rao S., D’Souza G., Dias M., et al. Evidence for the heterologous benefits of prior BCG vaccination on COVISHIELD™ vaccine-induced immune responses in SARS-CoV-2 seronegative young Indian adults. Front. Immunol. 2022;13:985938. doi: 10.3389/fimmu.2022.985938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y.P., Cai J.W., Liao L.J., Ding H., Cao X.J., Zhu G.D., Guo X.G. Effect of BCG Vaccination against SARS-CoV-2 Infection. Jpn. J. Infect. Dis. 2022;75:302–308. doi: 10.7883/yoken.JJID.2021.406. [DOI] [PubMed] [Google Scholar]
- 37.Gong W., Aspatwar A., Wang S., Parkkila S., Wu X. COVID-19 pandemic: SARS-CoV-2 specific vaccines and challenges, protection via BCG trained immunity, and clinical trials. Expert Rev. Vaccines. 2021;20:857–880. doi: 10.1080/14760584.2021.1938550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.BCG Vaccine for Health Care Workers as Defense against COVID-19 (BADAS) [(accessed on 15 November 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04348370.
- 39.Pittet L.F., Messina N.L., Gardiner K., Orsini F., Abruzzo V., Bannister S., Bonten M., Campbell J.L., Croda J., Dalcolmo M., et al. BCG vaccination to reduce the impact of COVID-19 in healthcare workers: Protocol for a randomised controlled trial (BRACE trial) BMJ Open. 2021;11:e052101. doi: 10.1136/bmjopen-2021-052101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clinical Trial Evaluating the Effect of BCG Vaccination on the Incidence and Severity of SARS-CoV-2 Infections among Healthcare Professionals during the COVID-19 Pandemic in Poland. [(accessed on 15 November 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04648800.
- 41.Use of BCG Vaccine as a Preventive Measure for COVID-19 in Health Care Workers (ProBCG) [(accessed on 15 November 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04659941.
- 42.Chang A.Y., Aaby P., Avidan M.S., Benn C.S., Bertozzi S.M., Blatt L., Chumakov K., Khader S.A., Kottilil S., Nekkar M., et al. One vaccine to counter many diseases? Modeling the economics of oral polio vaccine against child mortality and COVID-19. Front. Public Health. 2022;10:967920. doi: 10.3389/fpubh.2022.967920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yagovkina N.V., Zheleznov L.M., Subbotina K.A., Tsaan A.A., Kozlovskaya L.I., Gordeychuk I.V., Korduban A.K., Ivin Y.Y., Kovpak A.A., Piniaeva A.N., et al. Vaccination With Oral Polio Vaccine Reduces COVID-19 Incidence. Front Immunol. 2022;13:907341. doi: 10.3389/fimmu.2022.907341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.World Health Organization (WHO) Bacille Calmette-Guérin (BCG) Vaccination and COVID-19: Scientific Brief 12 April 2020. [(accessed on 20 November 2022)]; Available online: https://apps.who.int/iris/bitstream/handle/10665/331745/WHO-2019-nCoV-Sci_Brief-BCGvaccination-2020.1-eng.pdf?sequence=1&isAllowed=y.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during this investigation are available upon reasonable request from the corresponding author.