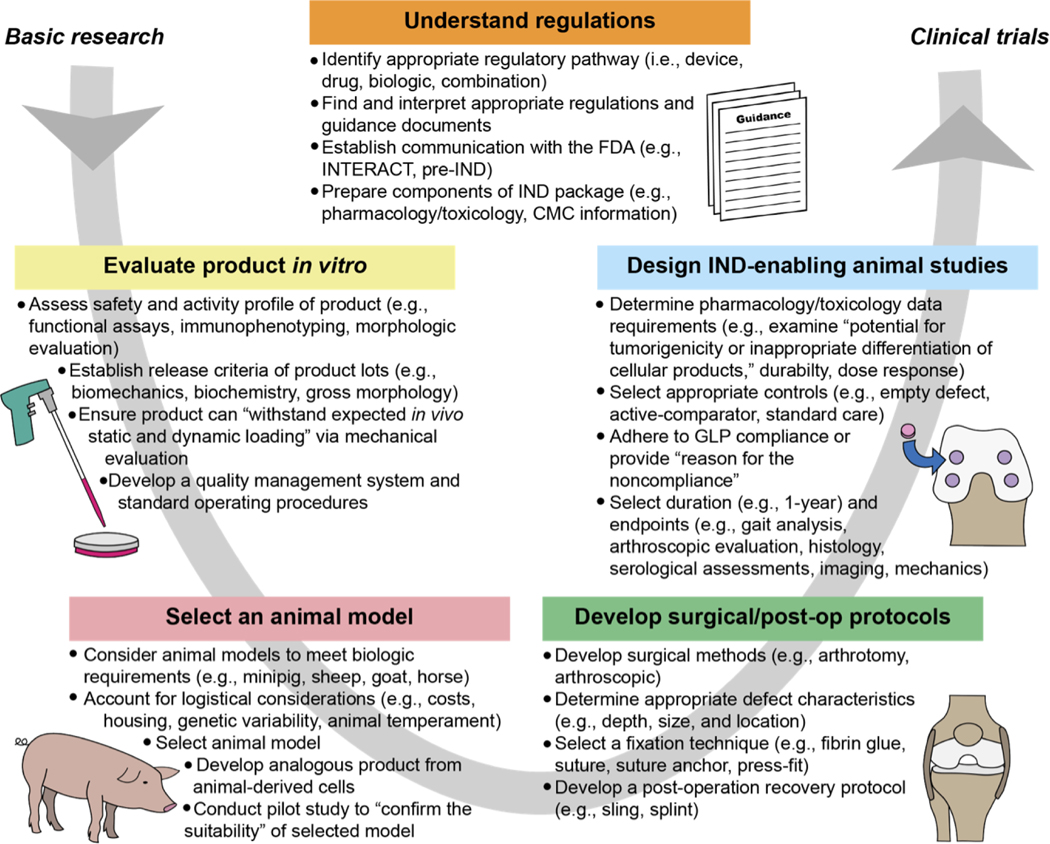
Fig. 1. Bottlenecks in translating biologic cartilage repair products from basic research to clinical trials.
Researchers must navigate the many challenges within this process at the stages of in vitro research, animal model selection, surgical development, and the conduct of IND-enabling animal studies. All stages should be conducted in accordance with appropriate regulations and guidance. This figure captures the salient aspects of this process, but for additional information guidance documents should be consulted. All quotations within this figure were taken from guidance documents (2, 3). Abbreviations: INitial Targeted Engagement for Regulatory Advice on CBER producTs (INTERACT), Chemistry, Manufacturing, and Controls (CMC), Good Laboratory Practice (GLP), Investigational New Drug (IND).