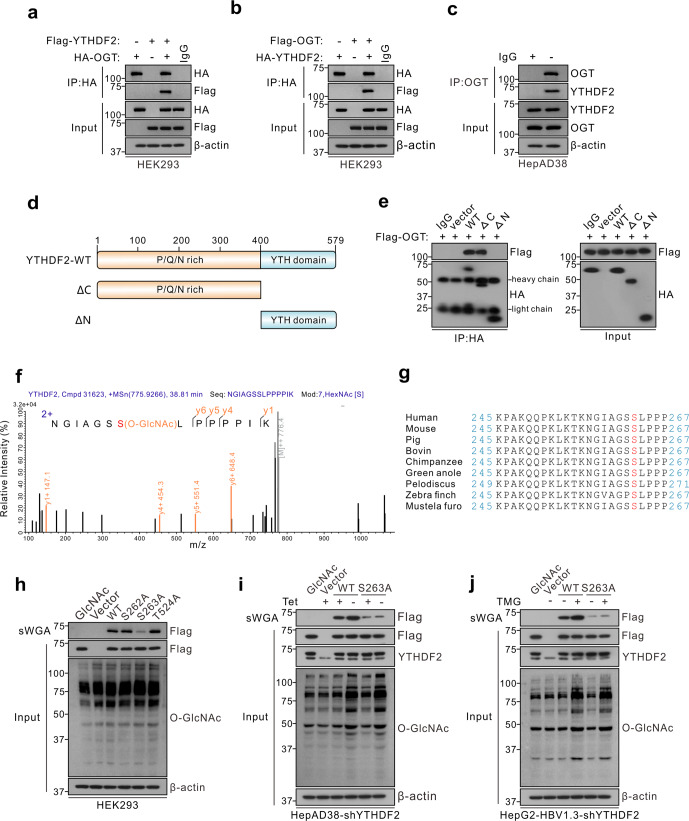
Fig. 2.
OGT mediates O-GlcNAcylation of YTHDF2 on Ser263. a, b Co-IP of OGT and YTHDF2 with anti-HA in HEK293 cells co-transfected with Flag (or HA)-YTHDF2 and HA (or Flag)-OGT expression constructs. c Co-IP of endogenous OGT and YTHDF2 in HepAD38 cells. d Schematic representation of the YTHDF2 constructs. YTHDF2-WT contains two domains, amino-terminal region (1–400aa, ΔC) and YTH domain (401–579aa, ΔN). e The interactions between OGT and the full-length or the truncated YTHDF2 (ΔC or ΔN) were determined in HEK293 cells by Co-IP. f LC-MS analysis of Flag-YTHDF2 identified residue Ser263 as the YTHDF2 O-GlcNAcylation site. g Cross-species sequence alignment of YTHDF2. h sWGA pull-down assay with anti-Flag in HEK293 cells. Cells were transfected with vectors encoding Flag-tagged YTHDF2 (WT, S262A, S263A or T524A). i, j YTHDF2 knockdown was performed in HepAD38 cells (i, with or without tet) or HepG2-HBV1.3 cells (j) by using lentiviral shRNA. Then cells were transfected with Flag-tagged YTHDF2 (WT or S263A), and subjected to sWGA pull-down assays. All the presented input was adjusted to a similar level for the following IP or sWGA-binding assay