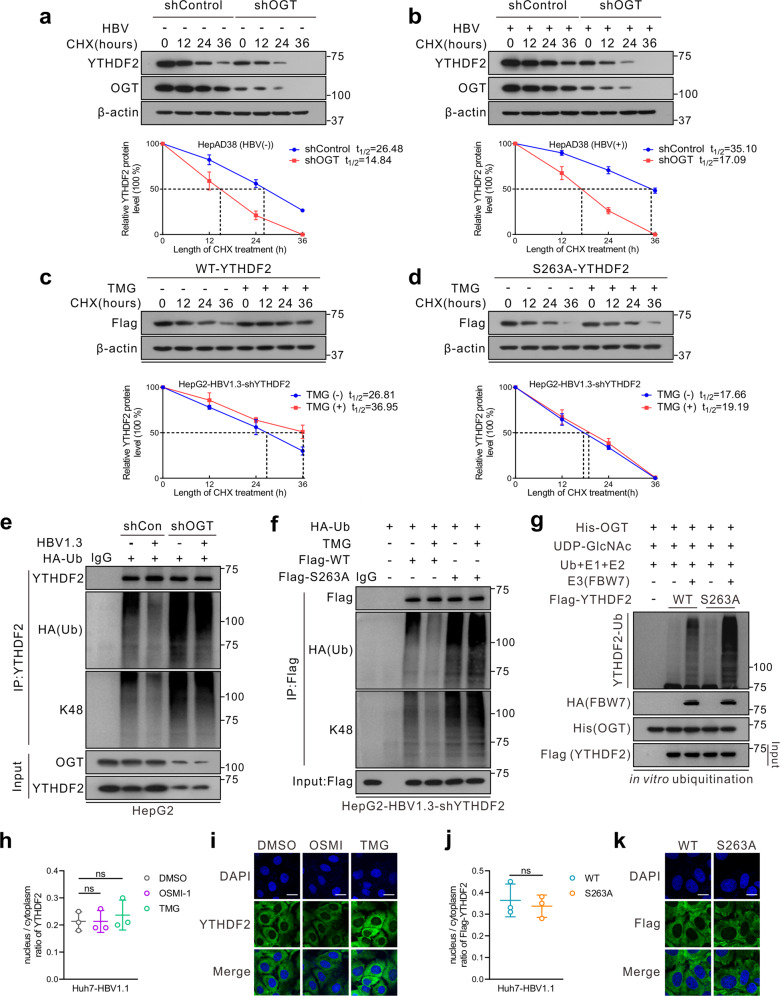
Fig. 3.
O-GlcNAcylation stabilizes YTHDF2 through suppression of its ubiquitination. a, b Half-life and quantitative analysis of YTHDF2 in HepAD38 cells treated with (a) or without (b) tet. Cells were transfected with OGT shRNA lentivirus and treated with 100 μM cycloheximide (CHX) for the indicated time. YTHDF2 levels were analyzed by immunoblotting (n = 3, performed in triplicate). c, d Half-life and quantitative analysis of Flag-YTHDF2 in HepG2-HBV1.3 cells treated with or without 25 μM TMG. HepG2 cells stably with YTHDF2 knockdown (shYTHDF2) were transfected with Flag-tagged YTHDF2-WT (c) or YTHDF2-S263A (d) and treated with 100 μM CHX (n = 3, performed in triplicate). The basal levels of Flag-YTHDF2 expression (WT or S263A) at 0 h were adjusted to a similar level for comparison. Data are expressed as mean ± SD in a–d. e, f In vivo YTHDF2 ubiquitination in HepG2 cells in the presence of HA-tagged ubiquitin (HA-Ub). e HepG2 cells were transfected with OGT shRNA lentiviral vector, with or without HBV infection; f HepG2-HBV1.3-shYTHDF2 cells were transfected with Flag-YTHDF2 (WT or S263A) and treated with 25 μM TMG. Cells were treated with 10 μM MG132 for 4 h to avoid degradation before harvest. After cell lysis, YTHDF2 was immunoprecipitated using anti-YTHDF2 or anti-Flag antibody. g In vitro YTHDF2 ubiquitination assay was performed with purified Flag-tagged WT or S263A YTHDF2, His-OGT, SCFFBW7 E3 complexes, E1, E2, Ub and UDP-GlcNAc. The reaction products were subjected to immunoblot analysis and YTHDF2 ubiquitination was detected with anti-YTHDF2. h–k Subcellular localization of YTHDF2 in Huh7 cells transfected with HBV1.1 (pCH9/3091, containing 1.1 copies of the HBV genome) (Huh7-HBV1.1) were determined by immunoblot analysis (h, j) or immunofluorescence staining (scale bars = 25 μm) (i, k). Nuclear and cytosolic fractions were immunoblotted with anti-YTHDF2 or anti-Flag, and the nucleus/cytoplasm ratio of YTHDF2 was quantified (n = 3, performed in triplicate) (h, j). Representative images for h and j were in Supplementary Fig. 3a, b. For h and i, Huh7-HBV1.1 cells were treated with 25 μM TMG or 20 μM OSMI-1 for 24 h; for j and k, Huh7-HBV1.1 cells with YTHDF2 knockdown (shYTHDF2) were transfected with Flag-YTHDF2 (WT or S263A)