Fig. 5. QCM-D analysis of Shh interactions with heparin.
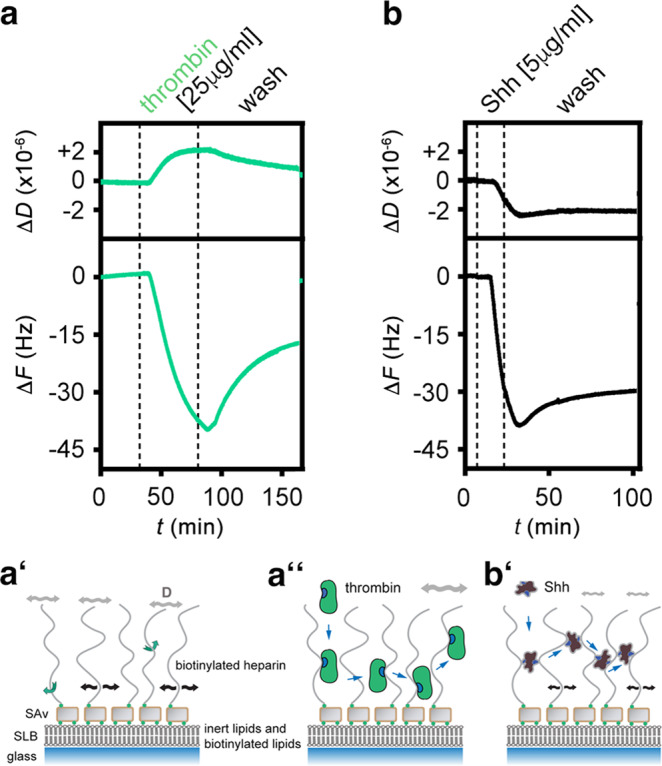
a–a″ Schematic representation and real-time analysis of thrombin binding to the functionalized QCM-D sensor surface. a′ represents the HS/heparin model matrix assembled on a silica surface. The supported lipid bilayer (SLB) exposes 5% biotinylated lipids that bind to streptavidin (SAv) linked to biotinylated heparin. Green arrows indicate free rotation of the coupled heparin chain, black arrows lateral movement on the SLB, and gray double-headed arrows indicate softness of the matrix (as sensed by increased dissipation, ΔD). a Thrombin binding decreases ΔF during the protein incubation step and increases ΔD correspondingly, demonstrating protein binding and retention of a relatively soft heparin/thrombin layer (a″). b, b′ Similar protein loading of the functionalized matrix (-ΔF about −40 Hz in both cases) by Shh is achieved more quickly (as indicated by faster ΔF decrease) and is associated with a negative ΔD, indicating that Shh rigidifies the heparin layer. The film rigidification is due to heparin cross-linking (as confirmed by complementary FRAP assays, see Supplementary Fig. 6c). Representative graphs of three independent experiments are shown.
