Figure 5.
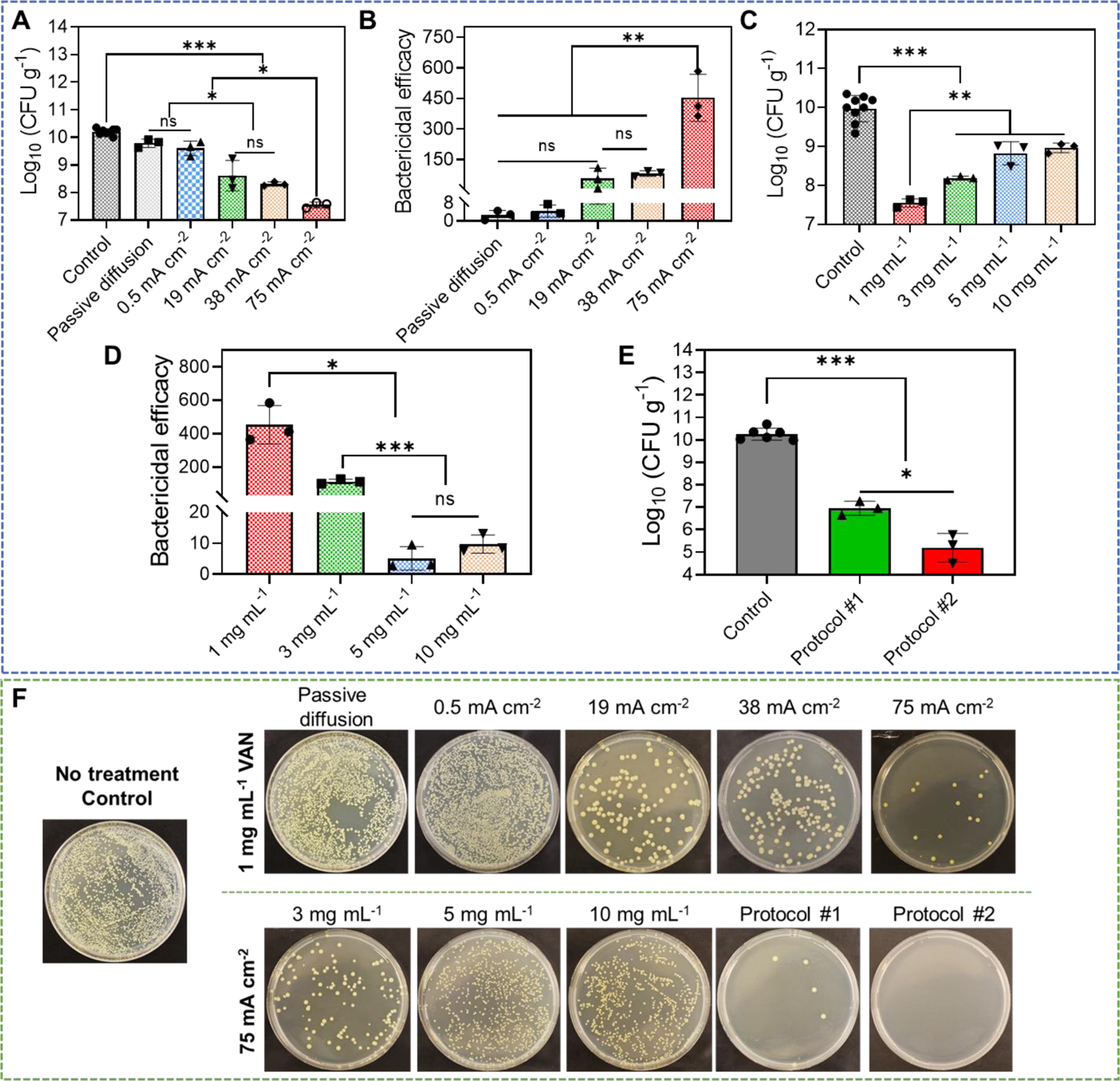
Anti-biofilm efficacy of our electrical biofilm treatment system combining the effects of high-intensity electrical debridement and iontophoretic VAN delivery. An ex vivo MRSA biofilm-infected porcine skin wound model was used in this study. (A) MRSA bacterial count (CFU g−1) in wound tissues measured at 24 h after biofilm treatment using our system. Treatment duration was 1 h. The drug chamber of the working device (anode) was loaded with 1 mg mL−1 VAN. Different current intensities were tested. Control received no treatment. Passive diffusion used 0 mA cm−2. (B) Bactericidal efficacy at 24 h after biofilm treatment calculated using data in (A). (C) MRSA bacterial count (CFU g−1) in wound tissues measured at 24 h after biofilm treatment using our system. Treatment duration was 1 h. The drug chamber of the working device (anode) was loaded with different concentrations of VAN. 75 mA cm−2 current intensity was tested. Control received no treatment. (D) Bactericidal efficacy at 24 h after biofilm treatment calculated using data in (C). (E) Anti-biofilm efficacy of repeated treatment protocols. Protocol #1 applied two treatments separated by 6 h. Protocol #2 applied two treatments separated by 24 h. Each treatment applied 75 mA cm−2 for 1 h using our biofilm treatment system. The drug chamber of the working device (anode) was loaded with 1 mg mL−1 VAN for all treatments. MRSA bacterial count (CFU g−1) in wound tissues was measured at 24 h after the last treatment. Control received no treatment. (F) Representative photographs of bacterial culture from wound tissues at 24 h after treatment (for single-treatment protocols) or after the last treatment (for repeated treatment protocols). All tissue homogenates were diluted 104 times with PBS before plating.
