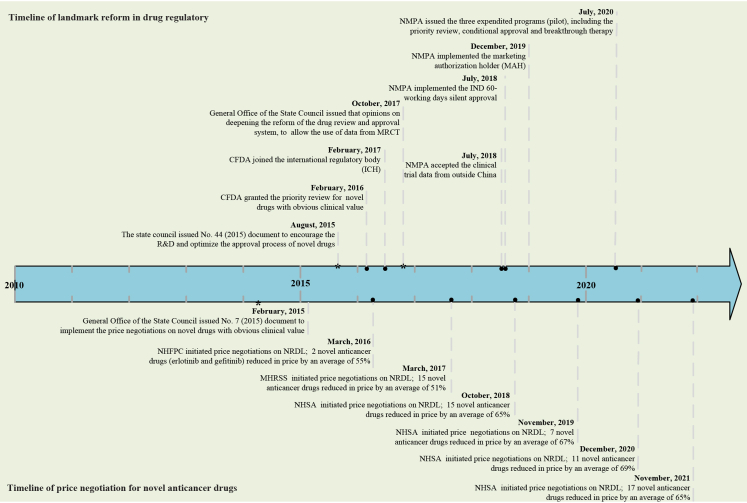
Fig. 1.
Timeline of landmark reform for drug regulatory and price negotiation for novel anticancer drugs in China. CFDA, China Food and Drug Administration (changed to the National Medical Products Administration in 2018); NMPA, National Medical Products Administration; IND, investigational new drug application; MRCT, multi-regional clinical trial; NHFPC, National Health and Family Planning Commission; MHRSS, Ministry of Human Resource and Social Security; NHSA, National Healthcare Security Administration; NRDL, National Reimbursement Drug List.