Abstract
A new Ag/Cu bimetallic cluster [Ag10Cu6(bdppthi)2(C≡CPh)12(EtOH)2](ClO4)4 (1, bdppthi = N,N′-bis(diphenylphosphanylmethyl)-tetrahydroimidazole) exhibited strong phosphorescent (PL) emission at 644 nm upon excitation at 400 nm. Removal of the coordinated EtOH molecules in 1 resulted in derivative 1a, which exhibited significant red-shifted emission at 678 nm. The structure and PL of 1 was restored on exposure to EtOH vapor. Cluster 1a also exhibited a vapor-chromic PL response towards other common organic solvent vapors including acetone, MeOH and MeCN. A PMMA film of 1a was developed as a reusable visible sensor for MeCN.
Keywords: photoluminescence material, vapor-chromic response, Ag/Cu complex, volatile organic vapor (VOC) detection
1. Introduction
Volatile organic compounds (VOCs) are hazardous air pollutants. Some coordination compounds can serve as photoluminescent (PL) probes for VOCs with a rapid and reversible switching or chromic response [1,2,3,4,5]. Coinage metal complexes have been developed with useful photophysical properties such as sensitive and selective emission color changes under external stimuli [6,7,8,9,10,11,12,13]. Bimetallic Ag/Cu complexes have attracted considerable attention in this respect due to their low cost and rich luminescence behaviors. For example, Chen et al. have reported an Ag(I)-Cu(I) complex showing reversible vapor-chromic phosphorescence, with the emission changing from bright yellow to green in response to THF or CHCl3 [14].
Vapor-chromic UV and PL responses of coordination compounds originate mostly from intramolecular structural distortion, such as the formation/disruption of metal–solvent bonds [15], molecular deformation [16,17] or conformational transformation [18,19]. The PL vapor-chromic response of metal complexes towards VOCs can be attributed to the interactions between the metal or their ligands and the VOC. In some cases, the small VOC molecules enter the lattice voids but do not participate in coordination bonds. There are only weak interactions, such as H-bonding, van der Waal’s forces or C-H···π or π···π interactions between these ‘free solvent molecules’ and the main structure that partly affects the complex’s energy levels [20,21,22,23,24,25,26,27,28]. In other cases, the VOC molecules coordinate with the metal ions to form metal–solvent bonds, which can significantly change the emission color or intensity [29,30,31,32]. For instance, Wang and co-workers have reported that the PL of an Au/Ag cluster reversibly shifted between green and yellow when the weakly ligated methanol molecules were removed or re-introduced [33].
Recently, we found that the strong emission of a Cu/Ag cluster [Ag10Cu6(bdppthi)2(C≡CPh)12(MeOH)2(H2O)](ClO4)4 (2, bdppthi = N,N′-bis(diphenylphosphanylmethyl)-tetrahydroimidazole) could be quenched by NH3, which enabled its use as a rapid, reversible and visual sensor [34]. During this quenching, the Ag10Cu6 cluster remained coordinated with MeOH and H2O molecules, while the NH3 only interacted with the MeOH ligands. Hence, we expected that the PL of this cluster would change when the MeOH and H2O molecules were removed or replaced by other small organic molecules, such as VOCs, that could coordinate with the Ag/Cu cluster core directly. We therefore prepared a new cluster [Ag10Cu6(bdppthi)2(C≡CPh)12(EtOH)2](ClO4)4 (1), in which the solvates were replaced with EtOH. The luminescence of 1 significantly red-shifted on elimination of EtOH to produce 1a, and was immediately restored upon exposure to EtOH. Other VOC vapors also resulted in a vapor-chromic PL response.
2. Results and Discussion
2.1. Synthesis and Characterization
2.1.1. Synthesis of 1
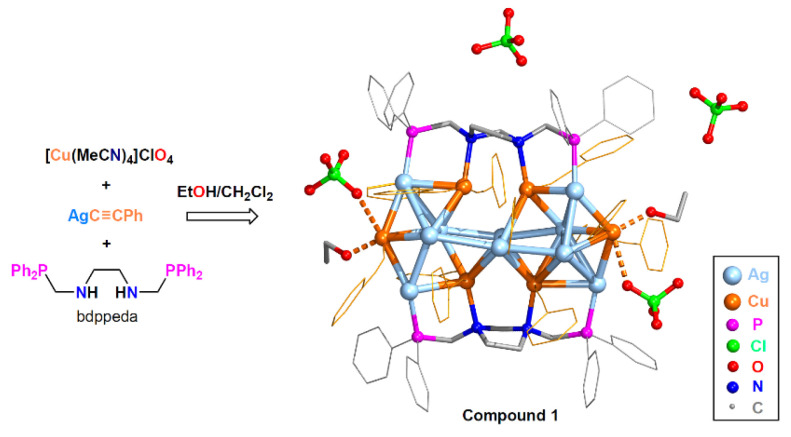
The reaction of N,N′-bis(diphenylphosphanylmethyl)ethylene diamine (bdppeda), [Cu(MeCN)4](ClO4) and AgC≡CPh (molar ratio 1:4:4) in CH2Cl2/EtOH (Figure 1) produced bimetallic cluster 1 in 73% yield. This methodology was similar to that used in the synthesis of compound 2, except for the solvent [34] and that the ligand bdppthi was generated in situ [35].
Figure 1.
Synthesis and structure of 1. The phenyl rings of -PPh2 groups and C≡CPh groups are plotted as gray and yellow hexagons. CH2Cl2 solvent molecules are omitted for clarity.
2.1.2. Single Crystal Structure of 1·2CH2Cl2
Single-crystal X-ray diffraction (SCXRD) of 1·2CH2Cl2 revealed that it crystallized in the monoclinic system P21/n space group. The asymmetric unit contained one [Ag10Cu6(bdppthi)2(C≡CPh)12(EtOH)2)]4+ tetracation, four ClO4− anions and two CH2Cl2 solvent molecules. As shown in Figure 1, the Ag10Cu6 cluster core may be viewed as two smaller Ag6Cu3 units joined by sharing two Ag(I) cations. The two bdppthi ligands stabilized and connected the two Ag6Cu2 units through four Ag-P and four Cu-N bonds. Each Cu(I) atom was coordinated oppositely by two C≡CPh anions. The average Ag-P, Cu-N and Cu-C bond lengths were 2.383(3), 2.271(9) and 1.915(13) Å, respectively. The Ag10Cu6 cores in compounds 1 and 2 were somewhat analogous. The Ag-Ag and Ag-Cu distances were in the range of 2.829–3.310 Å and 2.701–3.180 Å, respectively, indicating the presence of substantial metallophilic interactions [36]. Nevertheless, the metal–metal distances in 1 were slightly longer than those in 2. The smallest Cu-Cu distance of 2.969(2) Å (between Cu2 and Cu3) was larger than the sum of the van der Waals radii of the two Cu atoms (2.80 Å), which ruled out the existence of Cu-Cu interactions and revealed that the Ag10Cu6 cores in 1 and 2 were different. The two Cu(I) atoms at the end of the Ag10Cu6 cluster core were further coordinated with the O atoms of two EtOH molecules (Cu1-O1, 2.043(8) Å; Cu4-O2, 2.031(9) Å). These two Cu-O (EtOH) bond lengths were slightly shorter than the Cu-O (MeOH) (2.1134(1) and 2.2071(1) Å) and Cu-O (H2O) (2.1428(1) Å) interactions in 2. These differences in bond lengths demonstrated that the metallophilic interactions in the Ag10Cu6 core were weakened when EtOH molecules were closely coordinated to the end Cu ions. In addition, there were weak interactions between two of the ClO4− anions and these two Cu atoms: (Cu1-O5, 2.758(1) Å; Cu4-O9, 2.715(1) Å), while the other two ClO4− anions remained free.
2.1.3. Characterization of 1
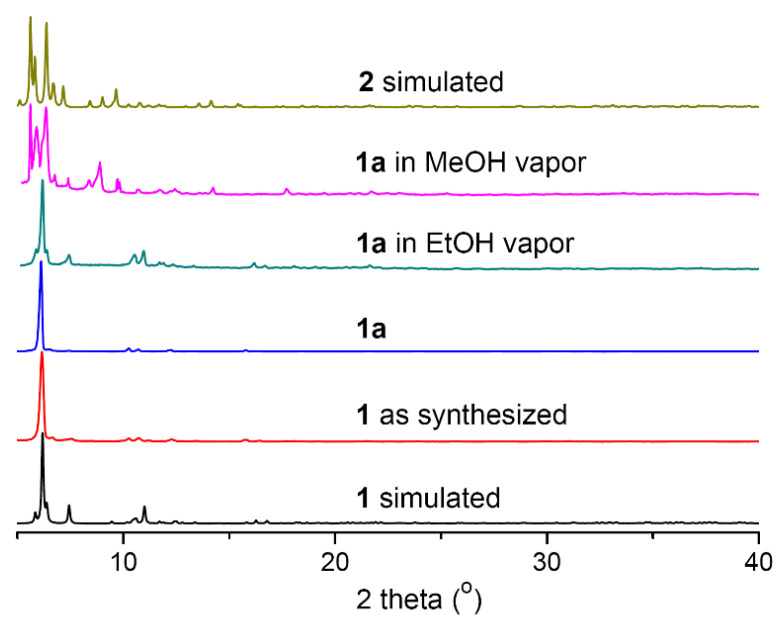
The CH2Cl2 molecules in 1·2CH2Cl2 readily escaped in air as evidenced by an absence of interaction in the solid-state structure. Thus, all other characterizations were performed on 1. This cluster readily dissolved in common organic solvents such as CH2Cl2, CHCl3, acetone, DMSO, DMF and MeCN, but was insoluble in Et2O, hexane and H2O. The elemental analysis of 1 was consistent with its molecular formula. The powder X-ray diffraction (PXRD) pattern of 1 correlated with the simulated spectra generated from the SCXRD data and was clearly different from that of 2 (Figure 2). The IR spectrum showed characteristic peaks at 2019 cm−1 for C≡C, 1082 cm−1 for ClO4−, and at 1483, 1435, 750, 687 and 619 cm−1 for the −Ph groups (Figure S1 (Supplementary Materials)). The positive-ion electrospray ion mass spectrometry (ESI-MS) of 1 (Figure S2) contained peaks attributed to [Ag2(bdppthi)(C≡CPh)]+ (m/z = 785.03) and [Ag(bdppthi)]+ (m/z = 575.09) cations and {[Ag10Cu4(C≡CPh)9(H2O)](ClO4)3+e+H+}2+ (m/z = 1278.89) dications, indicating the structure of 1 partly decomposed in the ESI-MS environment.
Figure 2.
PXRD patterns of compound 1, 1a in air, 1a in EtOH and MeOH vapors, and the simulated patterns of 1 and 2 from the SCXRD data.
2.1.4. Interconversions of 1 and 1a
Thermogravimetric analysis (TGA) of 1 in a N2 stream showed a weight loss of 2.2% between 120 and 150 °C, which matched the elimination of the two coordinated EtOH molecules (Calcd 2.24%) (Figure S3). We therefore treated 1 in vacuum by heating at 120 °C for 1 h and obtained its solventless derivative [Ag10Cu6(bdppthi)2(C≡CPh)12](ClO4)4 (1a). Cluster 1a was less crystalline than 1 and not suitable for SCXRD analysis. The weak IR vibration at 3420 cm–1 attributed to the −OH group of EtOH in 1 disappeared in 1a (Figure S1). As shown in Figure 2, the PXRD pattern of 1a was similar to that of 1, indicating its cell parameter and major structure remained unchanged. The elimination of some weak peaks might be due to the elimination of the EtOH molecules. When 1a was exposed to EtOH vapor for several minutes, the PXRD pattern fully recovered, indicating re-coordination of the EtOH molecules. However, when 1a was treated with MeOH vapor, the PXRD pattern resembled that of compound 2, indicating a phase-transition. This transition was reversible, so that desolvation of 2 and re-exposure to EtOH produced 1 quantitatively.
2.2. Photoluminescent Properties
2.2.1. Photoluminescent Properties of 1
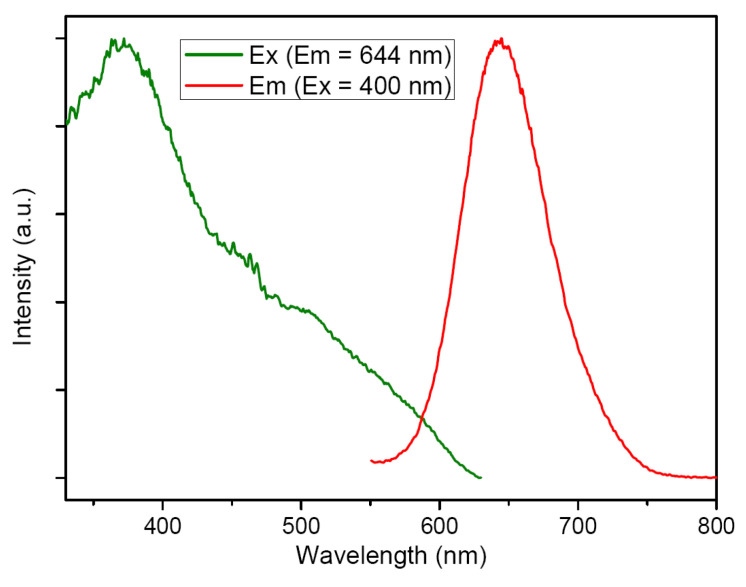
Upon excitation at 400 nm, crystals of 1 exhibited bright red emission at λmax = 644 nm (Figure 3) with a quantum yield (QY) of 10% at ambient temperature. The PL lifetime (τ, excited at 373 nm) was 7.48 μs. This relatively long lifetime and large Stokes shift (244 nm) suggested that this PL was a phosphorescent emission and likely arises from a metal cluster-centered triplet excited state modified by metal –metal interactions, mixed with a [C≡CPh→Ag10Cu6] 3LMCT transition [14,34]. The emission was not sensitive to excitation wavelengths, as the spectrum excited at 365 nm was similar to that excited at 400 nm except for a small intensity decay.
Figure 3.
Excitation and emission spectra of 1 at ambient temperature.
2.2.2. Photoluminescent Properties of 1a and Vapor-Chromic Responses toward Water and VOCs
Solid 1a emitted at λmax = 678 nm when excited at 400 nm (Figure 4). Compared to that of 1, this emission exhibited a 34 nm red-shift, reduced intensity (QY = 6%), and a slightly prolonged lifetime (τ = 8.82 us, excited at 373 nm). The emission of 1a could be fully restored to that of 1 (644 nm) after exposure to EtOH vapor, and this process showed good repeatability over four cycles (Figure S4). The PXRD of 1a remained steady during these cycles and for as long as one month later (Figure S5). We believe that during the interconversion of 1 and 1a, the departure and re-coordination EtOH molecules from the Cu atoms affect the electron density of the Ag10Cu6 cluster center, which influences the T1→S0 energy band, and therefore the shifting of the emission wavelength and intensity arises.
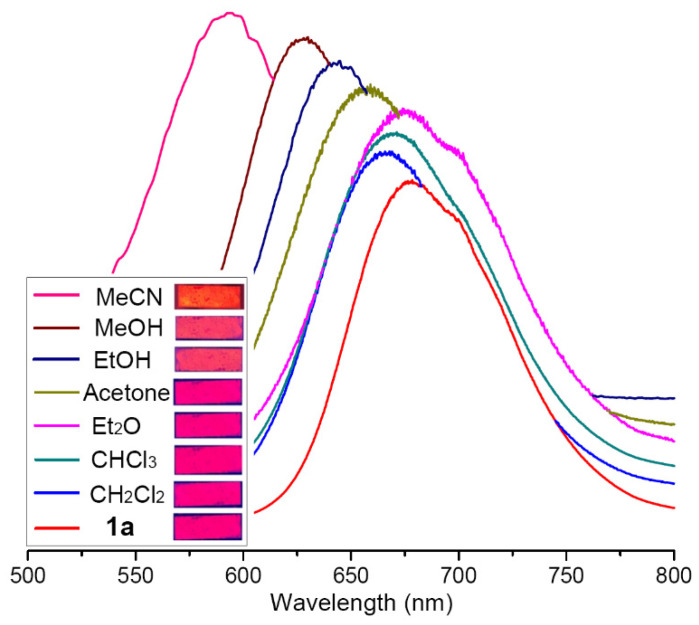
Figure 4.
Emission spectra of 1a and 1a on exposure to different VOC vapors upon excitation at 400 nm. Photos of the powdered 1a and 1a (coating on a quartz slice) on exposure to different VOC vapors under 365 nm excitation (inset).
Complex 1a was relatively stable toward air and moisture. The emission of 1a shifted to 718 nm when its powder was immersed in liquid water for 20 h (Figure S6). We suggested this red-shift was caused by the interaction between the Ag10Cu6 cluster center and water molecules. This interaction was weak and lessened when the content of water was decreased. Therefore, a low-energy shoulder could be observed at the emission curve of 1a, which indicated that a little portion of 1a was hydrated by moisture when it was put in open air for days.
The emission of 1a changed when this cluster was exposed to other VOC vapors. The less-coordinating VOC molecules, such as CH2Cl2, CHCl3 and Et2O, caused only minor blue-shifting (λmax = 666, 671 and 670 nm, respectively), whereas those VOCs with stronger donor groups, including acetone (C=O), MeOH (-OH) and MeCN (-CN), caused obvious blue-shifted emissions with λmax = 658 nm (acetone), 628 nm (MeOH) and 594 nm (MeCN), respectively. The changes in emission wavelength on exposure of 1a to MeOH and MeCN were 50 nm and 84 nm, respectively, which is visible to the naked eye under 365 nm LED irradiation (Figure 4).
2.3. Photoluminescent Probe for the Detection of MeCN
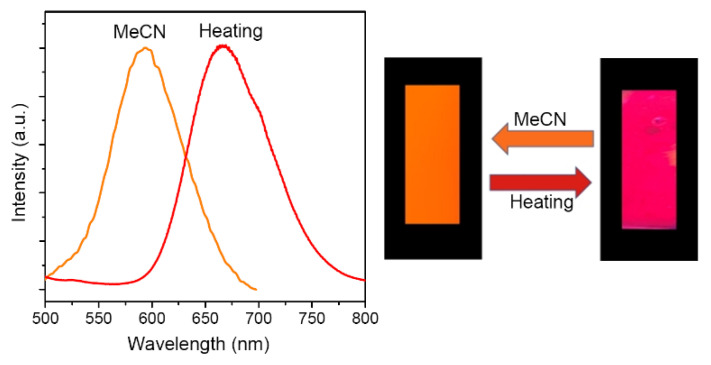
The significant vapor-chromic response of 1a encouraged us to prepare a PL sensing film by compositing 1a with PMMA (3% w/w). This film showed a visible red-to-orange PL change on exposure to a saturated atmosphere of MeCN vapor (Figure 5). The red emission could be recovered upon heating in air at 100 °C. This color interconversion was repeatable, giving a reusable PL probe for the detection of VOCs.
Figure 5.
Emission spectra (left, Ex = 400 nm) and photos (right, under 365 nm UV light) of 1a/PMMA film in MeCN vapor and heating to 100 °C.
3. Materials and Methods
3.1. Materials and Measurements
Bdppeda [37,38] and AgC≡CPh [39] were prepared by literature procedures. [Cu(MeCN)4](ClO4) was commercially available. Elemental analyses (C, H and N) were performed on a Carlo Erba CHNO-S microanalyzer. PXRD measurements were recorded on a Bruker D2 Phaser X-ray diffractometer with a Cu Kα source (30 kV, 10 mA). IR spectra were obtained on a VERTEX 70 FT-IR spectrometer (4000–500 cm−1) with an ATR probe. Thermogravimetric analysis (TGA) was completed on a TA SDT-600 analyzer under an N2 atmosphere in the range from room temperature to 900 °C, with a temperature heating rate of 10 °C/min. PL measurements were performed on an Edinburgh FLS1000 spectrophotometer. Positive-ion electrospray ion mass spectrometry (ESI-MS) was recorded on a Bruker microTOF-Q III mass spectrometer using MeOH as the mobile phase.
3.2. Synthesis of 1 and 1a
A mixture containing bdppeda (46 mg, 0.1 mmol), [Cu(MeCN)4](ClO4) (131 mg, 0.4 mmol), 5 mL of CH2Cl2 and 5 mL of EtOH was stirred for 0.5 h at ambient temperature. AgC≡CPh (84 mg, 0.4 mmol) was added into the resulting colorless solution and stirred for 3 h. The mixture turned red and was subsequently centrifuged. The supernatant was diffused with Et2O and afforded red crystals of 1·2CH2Cl2 after 1 day. The CH2Cl2 solvate escaped quickly in air, leaving 1 within an hour. Yield for 1: 117 mg (73% based on Ag). Anal. Calcd for C158H132Ag10Cl4Cu6N4O18P4: C, 46.28; H, 3.24; N, 1.37. Found: C, 45.82; H, 3.34; N, 1.26. IR (ATR, cm−1): 3419 (w), 3059 (w), 2019 (m), 1483 (m), 1435 (m), 1082 (vs), 750 (s), 687 (vs), 619(s).
Complex 1 was placed in a vacuum oven at 120 °C for 1 h and produced 1a on cooling to room temperature. The yield was almost quantitative.
3.3. Preparation of 1a/PMMA
A 9 mg sample of 1a was carefully ground in a mortar and pestle, dispersed in PMMA/toluene solution (20% w/w, 1.5 g) and sonicated for 30 min. The mixture was applied to glass slides (3 × 6 cm2) and left to dry in air over several hours. The dried film was removed from the slide and cut into small pieces (1.5 × 4 cm2).
3.4. Single-Crystal Crystallography
A single crystal of 1·2CH2Cl2 was collected from the above synthesis. SCXRD analysis was performed on a Bruker D8 Venture diffractometer using graphite-monochromated Ga Kα (λ = 1.34138 Å) radiation at 120 K. All data were integrated with Bruker SAINT and a multi-scan absorption correction was applied. The structure was solved by direct methods using SHELXS 2016/6 (Sheldrick, 2016) and refined by full-matrix least-squares methods against F2 by SHELXL-2016/6 (Sheldrick, 2016) [40]. All non-hydrogen atoms were refined anisotropically. The hydrogen atoms of the -OH groups of EtOH were first located from a Fourier map and then refined to ride on the O atoms. All other hydrogen atoms were added in idealized positions and constrained to ride on their parent atoms. The data were deposited to the Cambridge Crystallographic Data Centre (CCDC number 2226387). A summary of the key crystallographic data is given in Table 1. Selected bond lengths and angles are listed in Table S1.
Table 1.
Selected crystallographic data and refinement parameters for 1·2CH2Cl2.
| Compound | 1·2CH2Cl2 |
|---|---|
| Empirical formula | C160H136Ag10Cl8Cu6N4O18P4 |
| Formula weight | 4270.14 |
| Crystal system | monoclinic |
| Space group | P21/n |
| a/Å | 30.834(2) |
| b/Å | 16.5897(11) |
| c/Å | 33.5688(19) |
| β/° | 116.652(2) |
| V/Å3 | 15,346.8(17) |
| Z | 4 |
| ρcalc/g·cm−3 | 1.848 |
| μ/mm−1 | 12.665 |
| F(000) | 8432 |
| θmax/° | 56.966 |
| No. of reflections measured | 345,696 |
| No. of independent reflections | 31,366 (Rint = 0.1724) |
| Data/restraints/parameters | 31,366/79/1867 |
| R1 [I > 2.00 σ(I)] a | 0.1049 |
| wR2 (all reflections) | 0.3419 |
| Goodness of fit | 1.209 |
aR1 = Σ||Fo|–|Fc||/Σ|Fo|, wR2 = {Σw(Fo2 – Fc2)2/Σw(Fo2)2}1/2, GOF = {Σw((Fo2 – Fc2)2)/(n – p)}1/2, where n = number of reflections and p = total number of parameters refined.
4. Conclusions
An Ag10Cu6 cluster 1 stabilized by PNNP ligand bdppthi and C≡CPh anions was prepared. The two Cu ends of the Ag10Cu6 core were coordinated with EtOH molecules. Removing these solvates from 1 by vacuum heating produced 1a, which could be restored to 1 by exposure to EtOH vapor. The maximum PL emission of 1 at 644 nm shifted to 678 nm when converted to 1a. This reversible vapor-chromic response also occurred with other VOCs, particularly those with polar functional groups such as MeCN and MeOH, which exhibited the largest blue-shift of emissions up to 50 and 84 nm, respectively. A reusable chromic PL probe containing 3% (w/w) 1a in PMMA exhibited a visible red-to-orange emission change when exposed to MeCN vapor.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28031257/s1, Figure S1: IR spectra of 1 and 1a; Figure S2: Experimental and simulated isotopic pattens in the positive ion ESI-MS spectra of 1 in MeOH; Figure S3: TGA curve of 1 in a N2 stream; Figure S4: Emission of 1a over four cycles of exposure to EtOH vapor and vacuum heating; Figure S5: PXRD patterns of as-synthesized 1a, 1a after four EtOH exposure/elimination cycles and being left in air for another 1 month; Figure S6: Emission spectra of 1a in air and its powder after immersed in water for 20 h.; Table S1: Selected bond lengths and angles for 1·2CH2Cl2.
Author Contributions
W.Y., S.H., Y.W., S.Y. and X.-Q.C. conceived the study; Z.-G.R., H.-X.L. and D.J.Y. drafted the manuscript; Z.-G.R., W.Y. and Y.W. prepared the figures. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The crystallographic data are available from the Cambridge Crystallographic Data Centre (CCDC). Other data not presented in the Supplementary Materials are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was financially supported by the Suzhou Science and Technology Plan Project (Grant No. SGC2021016) and the National Natural Science Foundation of China (Grant No. 21671144).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Wenger O.S. Vapochromism in organometallic and coordination complexes: Chemical sensors for volatile organic compounds. Chem. Rev. 2013;113:3686–3733. doi: 10.1021/cr300396p. [DOI] [PubMed] [Google Scholar]
- 2.Lin H., Jang M., Suslick K.S. Preoxidation for colorimetric sensor array detection of VOCs. J. Am. Chem. Soc. 2011;133:16786–16789. doi: 10.1021/ja207718t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prodi L., Bolletta F., Montalti M., Zaccheroni N. Luminescent chemosensors for transition metal ions. Coord. Chem. Rev. 2000;205:59–83. doi: 10.1016/S0010-8545(00)00242-3. [DOI] [Google Scholar]
- 4.Zhang X., Li B., Chen Z.H., Chen Z.N. Luminescence vapochromism in solid materials based on metal complexes for detection of volatile organic compounds (VOCs) Mater. Chem. 2012;22:11427–11441. doi: 10.1039/c2jm30394a. [DOI] [Google Scholar]
- 5.Li E., Jie K., Liu M., Sheng X., Zhu W., Huang F. Vapochromic crystals: Understanding vapochromism from the perspective of crystal engineering. Chem. Soc. Rev. 2020;49:1517–1544. doi: 10.1039/c9cs00098d. [DOI] [PubMed] [Google Scholar]
- 6.Krytchankou I.S., Koshevoy I.O., Gurzhiy V.V., Pomogaev V.A., Tunik S.P. Luminescence solvato- and vapochromism of alkynyl-phosphine copper clusters. Inorg. Chem. 2015;54:8288–8297. doi: 10.1021/acs.inorgchem.5b00239. [DOI] [PubMed] [Google Scholar]
- 7.Jin M., Sumitani T., Sato H., Seki T., Ito H. Mechanical-stimulation-triggered and solvent-vapor-induced reverse single-crystal-to-single-crystal phase transitions with alterations of the luminescence color. J. Am. Chem. Soc. 2018;140:2875–2879. doi: 10.1021/jacs.7b12455. [DOI] [PubMed] [Google Scholar]
- 8.England K.R., Lim S.H., Luong L.M.C., Olmstead M.M., Balch A.L. Vapoluminescent behavior and the single-crystal-to-single-crystal transformations of chloroform solvates of [Au2(μ-1,2-bis(diphenylarsino)ethane)2](AsF6)2. Chem. Eur. J. 2019;25:874–878. doi: 10.1002/chem.201804937. [DOI] [PubMed] [Google Scholar]
- 9.Chu A., Hau F.K.W., Yao L.Y., Yam V.W.W. Decanuclear gold(I) sulfido pseudopolymorphs displaying stimuli-responsive RGBY luminescence changes. ACS Mater. Lett. 2019;1:277–284. doi: 10.1021/acsmaterialslett.9b00175. [DOI] [Google Scholar]
- 10.Artem’ev A.V., Davydova M.P., Berezin A.S., Ryzhikov M.R., Samsonenko D.G. Dicopper(I) paddle-wheel complexes with thermally activated delayed fluorescence adjusted by ancillary ligands. Inorg. Chem. 2020;59:10699–10706. doi: 10.1021/acs.inorgchem.0c01171. [DOI] [PubMed] [Google Scholar]
- 11.Smith M.B. The backbone of success of P,N-hybrid ligands: Some recent developments. Molecules. 2022;27:6293. doi: 10.3390/molecules27196293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baranov A.Y., Slavova S.O., Berezin A.S., Petrovskii S.K., Samsonenko D.G., Bagryanskaya I.Y., Fedin V.P., Grachova E.V., Artem’ev A.V. Controllable synthesis and luminescence behavior of tetrahedral Au@Cu4 and Au@Ag4 clusters supported by tris(2-pyridyl)phosphine. Inorg. Chem. 2022;61:10925–10933. doi: 10.1021/acs.inorgchem.2c01474. [DOI] [PubMed] [Google Scholar]
- 13.Huang Q., Zhang R., He L.-H., Chen J.-L., Zhano F., Liu S.-J., Wen H.-R. Thermo-, mechano-, and vapochromic dinuclear cuprous-emissive complexes with a switchable CH3CN−Cu bond. Inorg. Chem. 2022;61:15629–15637. doi: 10.1021/acs.inorgchem.2c02506. [DOI] [PubMed] [Google Scholar]
- 14.Xu L.J., Zhang X., Wang J.Y., Chen Z.N. High-efficiency solution-processed OLEDs based on cationic Ag6Cu heteroheptanuclear cluster complexes with aromatic acetylides. J. Mater. Chem. C. 2016;4:1787–1794. doi: 10.1039/C5TC03886C. [DOI] [Google Scholar]
- 15.Chen K., Shearer J., Catalano V.J. Subtle modulation of Cu4X4L2 phosphine cluster cores leads to changes in luminescence. Inorg. Chem. 2015;54:6245–6256. doi: 10.1021/acs.inorgchem.5b00443. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y., Wang J.Y., Zhang L.Y., Xu L.J., Chen Z.N. Vapor-triggered green-to-yellow luminescence conversion due to the variation of ligand orientations in tetranuclear copper(I) complex. Inorg. Chem. 2020;59:17415–17420. doi: 10.1021/acs.inorgchem.0c02707. [DOI] [PubMed] [Google Scholar]
- 17.Mizukami S., Houjou H., Sugaya K., Koyama E., Tokuhisa H., Sasaki T., Kanesato M. Fluorescence color modulation by intramolecular and intermolecular π−π interactions in a helical zinc(II) complex. Chem. Mater. 2005;17:50–56. doi: 10.1021/cm049744s. [DOI] [Google Scholar]
- 18.Kritchenkov I.S., Gitlina A.Y., Koshevoy I.O., Melnikov A.S., Tunik S.P. Luminescent silver–copper “hourglass” hepta- and decanuclear alkynyl-phosphine clusters. Eur. J. Inorg. Chem. 2018;2018:3822–3828. doi: 10.1002/ejic.201800631. [DOI] [Google Scholar]
- 19.Zhang L.Y., Xu L.J., Zhang X., Wang J.Y., Li J., Chen Z.N. Spectroscopic and phosphorescent modulation in triphosphine-supported PtAg2 heterotrinuclear alkynyl complexes. Inorg. Chem. 2013;52:5167–5175. doi: 10.1021/ic4000457. [DOI] [PubMed] [Google Scholar]
- 20.Laguna A., Lasanta T., López-de-Luzuriaga J.M., Monge M., Naumov P., Olmos M.E. Combining aurophilic interactions and halogen bonding to control the luminescence from bimetallic gold−silver clusters. J. Am. Chem. Soc. 2010;132:456–457. doi: 10.1021/ja909241m. [DOI] [PubMed] [Google Scholar]
- 21.Mo L.Q., Jia J.H., Sun L.J., Wang Q.M. Solvent-induced intercluster rearrangements and the reversible luminescence responses in sulfide bridged gold(I)–silver(I) clusters. Chem. Commun. 2012;48:8691–8693. doi: 10.1039/c2cc33446a. [DOI] [PubMed] [Google Scholar]
- 22.Lei Z., Pei X.L., Jiang Z.G., Wang Q.M. Cluster linker approach: Preparation of a luminescent porous framework with NbO topology by linking silver ions with gold(I) clusters. Angew. Chem. Int. Ed. 2014;53:12771–12775. doi: 10.1002/anie.201406761. [DOI] [PubMed] [Google Scholar]
- 23.Tzeng B.C., Lin C.Y., Hung J.W., Chen S.Y., Chang A.H.H., Lee G.H. Solvent-induced luminescence and structural transformation of a dinuclear gold(I) (aza-18-crown-6)dithiocarbamate compound. Inorg. Chem. 2021;60:2694–2703. doi: 10.1021/acs.inorgchem.0c03571. [DOI] [PubMed] [Google Scholar]
- 24.Luong L.M.C., Olmstead M.M., Balch A.L. A non-luminescent polymorph of [(cyclohexyl isocyanide)2Au]PF6 that becomes luminescent upon grinding or exposure to dichloromethane vapor. Chem. Commun. 2021;57:793–796. doi: 10.1039/D0CC06161A. [DOI] [PubMed] [Google Scholar]
- 25.Jiang B., Zhang J., Ma J.Q., Zheng W., Chen L.J., Sun B., Li C., Hu B.W., Tan H., Li X., et al. Vapochromic behavior of a chair-shaped supramolecular metallacycle with ultra-stability. J. Am. Chem. Soc. 2016;138:738–741. doi: 10.1021/jacs.5b11409. [DOI] [PubMed] [Google Scholar]
- 26.Hudson Z.M., Sun C., Harris K.J., Lucier B.E.G., Schurko R.W., Wang S. Probing the structural origins of vapochromism of a triarylboron-functionalized platinum(II) acetylide by optical and multinuclear solid-state NMR spectroscopy. Inorg. Chem. 2011;50:3447–3457. doi: 10.1021/ic102349h. [DOI] [PubMed] [Google Scholar]
- 27.Nayeri S., Jamali S., Jamjah A., Samouei H. Tetranuclear Au2Cu2 clusters with butterfly- and planar-shaped metal cores: Strong rigidochromism induced by Jahn–Teller distortion in two-coordinated gold(I) centers. Inorg. Chem. 2019;58:12122–12131. doi: 10.1021/acs.inorgchem.9b01414. [DOI] [PubMed] [Google Scholar]
- 28.López-de-Luzuriaga J.M., Monge M., Olmos M.E., Quintana J., Rodríguez-Castillo M. Stimuli-responsive solvatochromic Au(I)–Ag(I) clusters: Reactivity and photophysical properties induced by the nature of the solvent. Inorg. Chem. 2019;58:1501–1512. doi: 10.1021/acs.inorgchem.8b03022. [DOI] [PubMed] [Google Scholar]
- 29.Strasser C.E., Catalano V.J. “On−off” Au(I)⋯Cu(I) interactions in an Au(NHC)2 luminescent vapochromic sensor. J. Am. Chem. Soc. 2010;132:10009–10011. doi: 10.1021/ja104585q. [DOI] [PubMed] [Google Scholar]
- 30.Li Y.J., Deng Z.Y., Xu X.F., Wu H.B., Cao Z.X., Wang Q.M. Methanol triggered ligand flip isomerization in a binuclear copper(I) complex and the luminescence response. Chem. Commun. 2011;47:9179–9181. doi: 10.1039/c1cc12857d. [DOI] [PubMed] [Google Scholar]
- 31.Lee J.Y., Kim H.J., Jung J.H., Sim W., Lee S.S. Networking of calixcrowns: From heteronuclear endo/exocyclic coordination polymers to a photoluminescence switch. J. Am. Chem. Soc. 2008;130:13838–13839. doi: 10.1021/ja805337n. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y., Shi Y., Zou X., He Y., Wang X. Pyridylphosphine supported Ag(I) and Cu(I) complexes for detection of alcohols and nitriles via structural transformations from 1D to 0D. CrystEngComm. 2019;21:5595–5601. [Google Scholar]
- 33.Lei Z., Chang S.S., Wang Q.M. Vapochromic gold(I)–silver(I) cluster protected by alkynyl and phosphine ligands. Eur. J. Inorg. Chem. 2017;2017:5098–5102. doi: 10.1002/ejic.201701012. [DOI] [Google Scholar]
- 34.Wang Y., Yan J.J., Hu S., Young D.J., Li H.X., Ren Z.G. A photoluminescent Ag10Cu6 cluster stabilized by a PNNP ligand and phenylacetylides selectively and reversibly senses ammonia in air and water. Chem. Asian J. 2021;16:2681–2686. doi: 10.1002/asia.202100783. [DOI] [PubMed] [Google Scholar]
- 35.Sun S., Ren Z.G., Yang J.H., He R.T., Wang F., Wu X.Y., Gong W.J., Li H.X., Lang J.P. Formation of N-heterocyclic Biphosphine Ligands from Ag(I)-Assisted Condensation Reactions between bdppeda and Formaldehyde and Their Binuclear Silver(I) Complexes. Dalton Trans. 2012;41:8447–8454. doi: 10.1039/c2dt30860f. [DOI] [PubMed] [Google Scholar]
- 36.Bondi A. Van der Waals volumes and radii. J. Phys. Chem. 1964;68:441–451. doi: 10.1021/j100785a001. [DOI] [Google Scholar]
- 37.Zhang J., Vittal J.J., Henderson W., Wheaton J.R., Hall I.H., Hor T.S.A., Yan Y.K. Tricarbonylrhenium(I) complexes of phosphine-derivatized amines, amino acids and a model peptide: Structures, solution behavior and cytotoxicity. J. Organomet. Chem. 2002;650:123–132. doi: 10.1016/S0022-328X(02)01200-7. [DOI] [Google Scholar]
- 38.Grim S.O., Matienzo L.J. The synthesis and characterization of some novel polydentate phosphorus-nitrogen ligands. Tetrahedron Lett. 1973;14:2951–2953. doi: 10.1016/S0040-4039(01)96290-2. [DOI] [Google Scholar]
- 39.Teo K., Xu Y.H., Zhong B.Y., He Y.K., Chen H.Y., Qian W., Deng Y.J., Zou Y.H. A comparative study of third-order nonlinear optical properties of silver phenylacetylide and related compounds via ultrafast optical Kerr effect measurements. Inorg. Chem. 2001;40:6794–6801. doi: 10.1021/ic010408c. [DOI] [PubMed] [Google Scholar]
- 40.Sheldrick G.M. SHELXTL-2016/6. Universität Göttingen; Göttingen, Germany: 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The crystallographic data are available from the Cambridge Crystallographic Data Centre (CCDC). Other data not presented in the Supplementary Materials are available on request from the corresponding author.