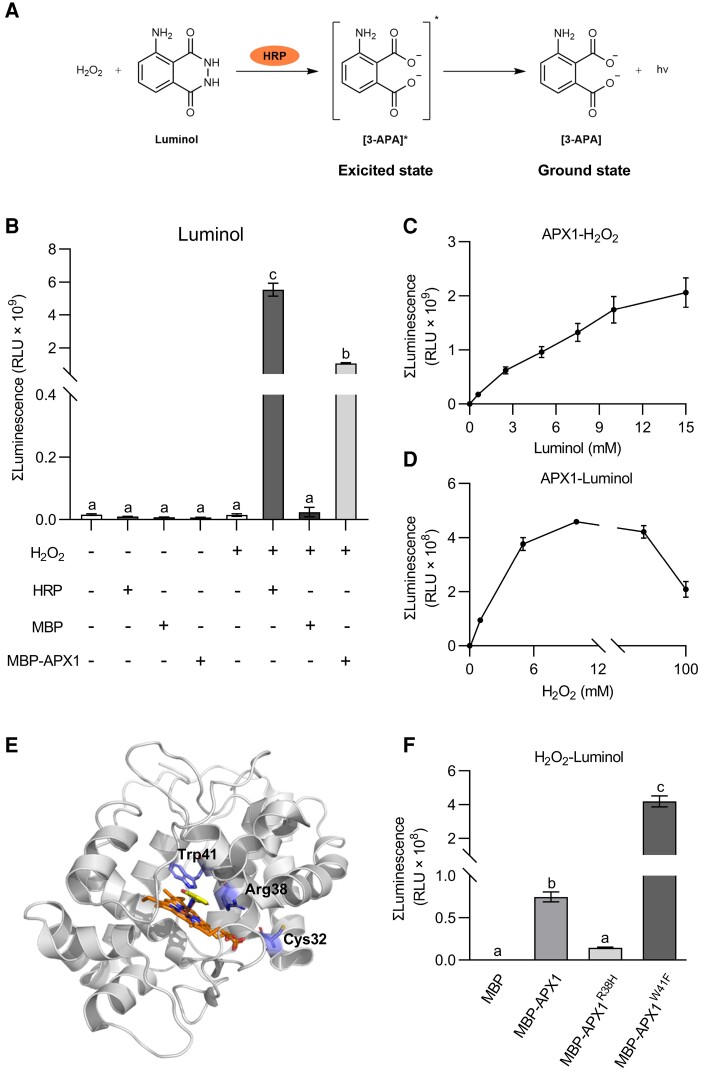
Figure 5.
APX1 catalyzes the luminol-H2O2 reaction in vitro. A, HRP catalyzes the luminol-H2O2 reaction. In the presence of HRP, luminol is oxidized by H2O2 to create an excited-state product, 3-aminophthalate (3-APA*), whose decay to the ground state results in photon emission. B, APX1 catalyzed the chemiluminescence reaction of luminol. MBP-tagged APX1 recombinant proteins were purified from E. coli. Luminol (300 μM) and MBP, MBP-APX1, or HRP (0.04 mg mL−1) were added first, and light signals were recorded immediately after the addition of H2O2 (10 mM). Values represent the total photon counts within 10 min. Data are shown as the mean ± SE (n = 4). Different letters above the bars indicate significant differences (P ≤ 0.05, one-way ANOVA). C, APX1 activity was corresponded with luminol concentrations below 15 mM. Data are shown as the mean ± SE (n = 5). D, APX1 activity corresponded with H2O2 concentrations below 10 mM. Data are represented as the mean ± SE (n = 6). E, Homology modeling of the structure of APX1 using the 3D coordination of the soybean cAPX1 (PDB-1V0H) as a template. The heme cofactor and substrate salicylhydroxamic acid locate in the middle. The 3 important residues, Cys32, Arg38 and Trp41, are marked F, Catalytic abilities of APX1 variants, APX1R38H and APX1W41F. Luminol (300 μM) and MBP, MBP-APX1, MBP-APX1R38H, or MBP-APX1W41F (0.09 mg mL−1) were added first, and light signals were recorded immediately after the addition of H2O2 (1 mM). Values represent the total photon counts within 10 min. Data are shown as the mean ± SE (n = 8). Different letters above the bars indicate significant differences (P ≤ 0.05, one-way ANOVA). All experiments were repeated 3 times with similar results.