Figure 3.
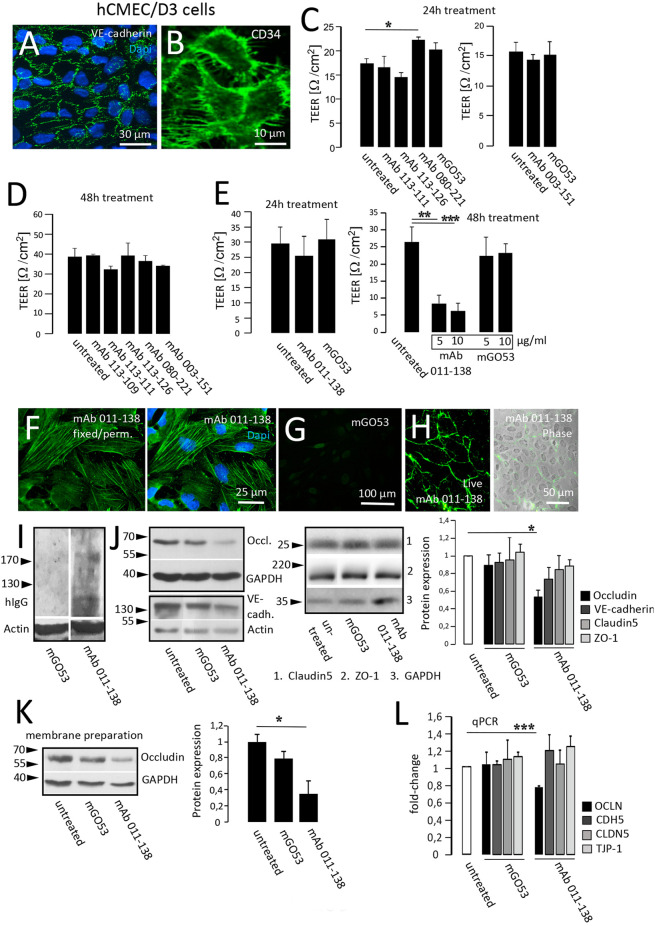
Antibody 011-138 decreases transendothelial electrical resistance and downregulates tight junctional Occludin. (A,B) Human cerebral microvascular endothelial cells (hCMEC/D3) were used as an in vitro blood-brain barrier model. Tight junction protein VE-cadherin and the endothelial cell marker CD34 were clearly expressed and depicted adjacent cell boundaries. The following functional experiments were carried out on live cells grown to confluence. (C) After 24 h of antibody treatment at 5 μg/ml, none of the human monoclonal antibodies (mAb) decreased TEER, only mAb 080-221 increased TEER. (D) No effects on TEER were observed after incubation for 48 h with 5 μg/ml with the respective antibodies. (E) Incubation with 5 μg/ml mAb 011-138 for 24 h had no significant effects on TEER. Conversely, incubation for 48 h resulted in a highly significant reduction of electrical resistance by mAb 011-138, but not mGO53, used as control Ab. Values analyzed in triplicates are expressed as means ± SEM from a representative experiment that was at least repeated once per condition (C–D) or from three independent experiments (E). (F–H) Immunofluorescence detection of mAb 011-138 binding (5 μg/ml) to either live or fixed and permeabilized hCMEC/D3 cells. Fixed cells revealed a filamentous signal of mAb 011-138, and live cells showed a rather surface localized signal. Incubation with mGO53 resulted in no signal under either condition (G, shown for fixed cells). (I) Western blotting of hCMEC/D3 cell homogenates with either mAb 011-138 or control mGo53. Bound monoclonal 011-138 expressed higher molecular weight bands with a prominent signal at or above 200 kDa, mGO53 revealed no immune signal. (J) Confluent hCMEC/D3 cells were incubated for 48 hwith 5 μg/ml mAb 011-138 or mGO53. Western blotting for detection of Occludin, VE-cadherin, Claudin5, and ZO-1 expression. GAPDH or Actin were used as loading control. Incubation with mAb 011-138 resulted in a significant downregulation of Occludin expression exclusively. Values adjusted to loading are expressed as means ± SEM from 4–5 independent experiments. (K) Membrane preparations were performed from hCMEC/D3 cells following incubation with 5 μg/ml mAb 011-138 or mGO53. Western blot analysis revealed a significant removal of Occludin from the membrane compartment largely consisting of plasma membrane fractions following incubation with mAb 011-138. Western blot values adjusted to loading are expressed as normalized means ± SEM from three independent experiments. (L) Additionally, quantitative RT-PCR was performed to check for alterations in gene regulation of tight junction proteins. Calnexin (CANX) was used as endogenous control. Significantly decreased mRNA levels were found for Occludin (OCLN), the other genes (CDH5 VE-cadherin; CLDN5 Claudin5, and TJP-1 ZO-1) remained unchanged. Quantitative PCR values are expressed as means ± SEM from three independent experiments. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.