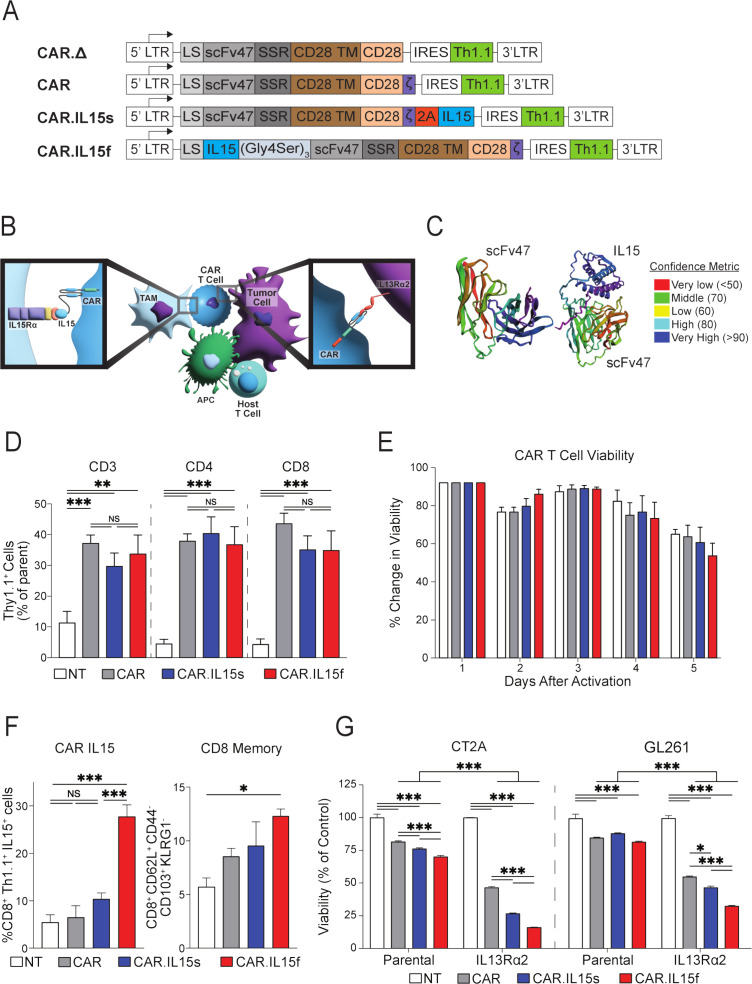
Figure 3.
CAR T cells modified to express IL15 successfully kill glioma cells in vitro. (A) CAR.Δ, CAR, CAR.IL15s and CAR.IL15f murine constructs were designed as described in the Materials and Methods section. (B) Schematic diagram shows potential interaction of CAR.IL15s and CAR.IL15f with glioma and cells of the tumor microenvironment. (C) Modeling of antigen-binding part of CAR protein, a scFvIL13Rα2 (clone 47) alone and as a fusion with IL15. (D) Flow cytometry analysis to quantify CAR T cell transduction efficiency using a fluorochrome congjugated antibody against Thy1.1 (n=3, **p<0.01, ***p<0.001). (E) Trypan blue exclusion assay was perfromed to determine viability of CAR, CAR.IL15s, and CAR.IL15f T cells. Values presented as % change in viability over 5 days postactivation. (F) Flow cytometry analysis of IL15 expression on the surface of CD8+Thy1.1+ CAR T cells (n=3, ***p<0.001), and analysis of the CAR T cells for their memory phenotype after vital transduction following 2 days of rest after retroviral transduction in vitro (n=3, *p<0.05). (G) Analysis of the viability of parental and IL13Rα2-expressing GL261 and CT2A cells in coculture with CAR T cells. Non-transduced (NT) T cells were used as a negative control (n=3, ***p<0.001). Viability values in each group were normalized to NT cells. The experiments was repeated two times. CAR, chimeric antigen receptor.