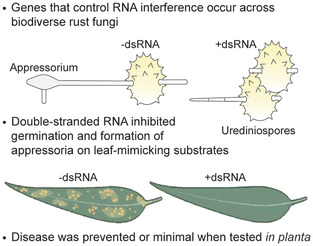
Abstract
Rust fungi (Pucciniales) are a diverse group of plant pathogens in natural and agricultural systems. They pose ongoing threats to the diversity of native flora and cause annual crop yield losses. Agricultural rusts are predominantly managed with fungicides and breeding for resistance, but new control strategies are needed on non‐agricultural plants and in fragile ecosystems. RNA interference (RNAi) induced by exogenous double‐stranded RNA (dsRNA) has promise as a sustainable approach for managing plant‐pathogenic fungi, including rust fungi. We investigated the mechanisms and impact of exogenous dsRNA on rust fungi through in vitro and whole‐plant assays using two species as models, Austropuccinia psidii (the cause of myrtle rust) and Coleosporium plumeriae (the cause of frangipani rust). In vitro, dsRNA either associates externally or is internalized by urediniospores during the early stages of germination. The impact of dsRNA on rust infection architecture was examined on artificial leaf surfaces. dsRNA targeting predicted essential genes significantly reduced germination and inhibited development of infection structures, namely appressoria and penetration pegs. Exogenous dsRNA sprayed onto 1‐year‐old trees significantly reduced myrtle rust symptoms. Furthermore, we used comparative genomics to assess the wide‐scale amenability of dsRNA to control rust fungi. We sequenced genomes of six species of rust fungi, including three new families (Araucariomyceaceae, Phragmidiaceae, and Skierkaceae) and identified key genes of the RNAi pathway across 15 species in eight families of Pucciniales. Together, these findings indicate that dsRNA targeting essential genes has potential for broad‐use management of rust fungi across natural and agricultural systems.
Keywords: dsRNA, fungal genomics, in vitro, myrtle rust, RNAi, rust fungi, spore germination
RNA interference genes are present in the Pucciniales and double‐stranded RNA targeting essential genes inhibits urediniospore germination and reduces disease symptoms.
1. INTRODUCTION
Rust fungi (Pucciniales) are the largest group of plant pathogens, impacting both agricultural and natural ecosystems (Aime & McTaggart, 2021). Agricultural rusts cause large‐scale crop yield losses, while environmentally significant rust diseases, such as myrtle rust (caused by Austropuccinia psidii) and white pine blister rust (caused by Cronartium ribicola), have caused population decline and localized extinction of endemic flora (Fensham & Radford‐Smith, 2021; Pegg et al., 2017; Schoettle & Sniezko, 2007). Common controls of rusts include synthetic fungicides (Miles et al., 2007), deployment of resistant genotypes (Babu et al., 2020; Glen et al., 2007; Periyannan, 2018), and breeding for resistance (Ellis et al., 2014; Varshney et al., 2007). However, these short‐term solutions are limited by evolving pathogens (Drenth et al., 2019; McDonald & Linde, 2002), financial and environmental costs associated with agrichemicals (Lykogianni et al., 2021; Makinson, 2018; Makinson & Conn, 2014; Pegg et al., 2014), and the time required to breed improved crop varieties (Almeida et al., 2021). These traditional control strategies are also not viable in the conservation of non‐crop species.
RNA interference (RNAi) is a conserved eukaryotic mechanism that regulates posttranscription expression of endogenous genes and defends cells against viruses and transposons (Baulcombe, 2004; Holoch & Moazed, 2015; Nakayashiki & Nguyen, 2008). The RNAi pathway is initiated when Dicer‐like (DCL) enzymes recognize and cleave double‐stranded RNA (dsRNA) into 20–24 nucleotide (nt) small interfering RNAs (siRNAs) or microRNAs (miRNAs). These small RNAs (sRNAs) associate with Argonaute (AGO) proteins to form the RNA‐induced silencing complex (RISC). The RISC recognizes and degrades complementary RNA, repressing transcription or translation of homologous DNA/RNA (Bologna & Voinnet, 2014; Torres‐Martinez & Ruiz‐Vázquez, 2017). In some cases, the process is amplified by RNA‐dependent RNA polymerases (RdRPs) that promote continuous silencing of targeted genes (Dalmay et al., 2000; Sijen et al., 2001). In the context of rust fungi, genes encoding the RNAi pathway, including AGO, DCLs, and RdRPs, have been studied primarily in wheat rusts, where they play roles in fungal growth, development, and pathogenicity (Dubey et al., 2020; Dutta et al., 2019; Kiran et al., 2016; Sperschneider et al., 2021). However, it is unknown whether genes encoding a functional RNAi pathway are present in all species of the Pucciniales.
RNAi has been adapted as a pathogen‐specific, non‐transgenic, pest and disease control with demonstrated effectiveness against viruses, insects, and fungal pathogens (Fletcher et al., 2020; Taning et al., 2020). RNAi could be used for crop protection either through a transgenic approach, known as host‐induced gene silencing (HIGS), or a nontransgenic approach, known as spray‐induced gene silencing (SIGS). In HIGS, host plants are genetically engineered to produce pest‐ or pathogen‐specific dsRNA, whereas in SIGS, dsRNA or siRNA are applied exogenously (Fletcher et al., 2020, Taning et al., 2020).
HIGS and SIGS have been used successfully against species in two families of rust fungi, Pucciniaceae and Phakopsoraceae, in both whole‐plant and detached leaf assays (Gill et al., 2018; Hu et al., 2020; Panwar et al., 2018; Qi et al., 2018; Saito et al., 2022; Yin et al., 2015; Zhu et al., 2017). Target genes have included those that encode haustorial proteins (Yin et al., 2015), kinases (Panwar et al., 2018; Qi et al., 2018; Zhu et al., 2017), acetyl‐transferases (Gill et al., 2018; Hu et al., 2020), and chitin synthases (Saito et al., 2022). Despite this success, the precise molecular mechanisms of HIGS and SIGS are not fully understood. It is hypothesized that dsRNA expressed in transgenic plants, that is, HIGS, is cleaved into siRNAs by plant DCLs and transferred from host to pathogen via extracellular vesicles, a mechanism known as cross‐kingdom RNAi (Cai et al., 2018; Rutter & Innes, 2018). In contrast, dsRNA molecules applied in SIGS are likely to be taken up directly by fungi on leaf surfaces (Šečić & Kogel, 2021). This is supported by studies in vitro (Qiao et al., 2021; Wytinck et al., 2020) and one study on soybean leaves by Saito et al. (2022), who found that dsRNA was more effective in reducing lesion formation on soybean leaves when it was co‐applied with Phakospora pachyrhizi urediniospores than when it was infiltrated into leaves prior to inoculation.
Spores of fungal foliar pathogens anchor themselves, germinate, and concentrate their cytoplasm in appressoria to build force and penetrate leaf surfaces through stomata (Wynn, 1975). The interface between leaves and fungal spores in plant–pathogen interactions has been examined on artificial leaf‐like surfaces made from polystyrene (Bernstein & Jones, 1969; Collins & Read, 1997; Dickinson, 1949; Wynn, 1975), silicone (Lee & Dean, 1994), and cellulose nanofibre (Saito et al., 2021). Artificial leaf‐like surfaces were used to assess the effect of environmental factors on pathogen infection (Doan & Leveau, 2015) and to study how dsRNA‐induced RNAi impacts the infection process of rust fungi (Saito et al., 2022).
We investigated the mechanisms, conservation, and functionality of RNAi in rust fungi to assess its utility in the management of plant disease. We aimed to determine (i) whether genes encoding the RNAi pathway are conserved across the Pucciniales, (ii) which life cycle stages of rust fungi could take up exogenous dsRNA, (iii) whether dsRNA treatment affects the physiology of spore germination and infection by rust fungi, and (iv) whether essential genes are suitable as universal targets for exogenous dsRNA‐mediated management of rust fungi. We provide experimental evidence of the internalization/external association of dsRNA with infective spore stages, evidence for a functional RNAi pathway in two rust species, and genomic analyses to support a hypothesis that the RNAi pathway is conserved across rust fungi. The outcomes of this research will inform the design and implementation of next‐generation management strategies to control plant diseases in agricultural systems as well as natural environments.
2. RESULTS
2.1. dsRNA either associates externally or is internalized by urediniospores
To determine whether rust spores can take up dsRNA from the environment, urediniospores and teliospores of A. psidii, and urediniospores of Coleosporium plumeriae were incubated with in vitro‐synthesized Cy3‐labelled green fluorescent protein (GFP) dsRNA on water agar or glass slides, and then viewed under a confocal fluorescence microscope. Micrococcal nuclease was used to remove excess external Cy3‐dsRNA, as in Qiao et al. (2021). Germinating urediniospores of A. psidii and C. plumeriae displayed Cy3 fluorescence 4–8 h after dsRNA application, indicating that dsRNA is either internalized or associates with the surface of the spore, which may protect it from micrococcal nuclease activity (Figures 1a,b,d–f and S1). Cy3 fluorescence was seen across multiple Z‐stacks, showing that the Cy3‐dsRNA was present at different focal planes within the spores, which supports that dsRNA is internalized rather than external (Figure S2). Germinating urediniospores of A. psidii only displayed Cy3 fluorescence in their germ tubes (Figure 1a,b), whereas Cy3 fluoresced in the urediniospores and germ tubes of C. plumeriae (Figure 1d–f). Cy3‐CTP‐treated C. plumeriae urediniospores, which acted as a negative control, only displayed a background fluorescence in their germ tubes that was differentiated from Cy3‐dsRNA fluorescence with fluorescence lifetime imaging microscopy (FLIM; Datta et al., 2020; Figure S1). Fluorescence was seen in urediniospores at very early stages of germination, indicating that dsRNA associates with urediniospores as soon as they germinate (Figure 1e,f). Teliospores were fluorescent before micrococcal nuclease treatment (Figure 1a) but not after (Figure 1c), indicating that dsRNA was not internalized or externally associated.
FIGURE 1.
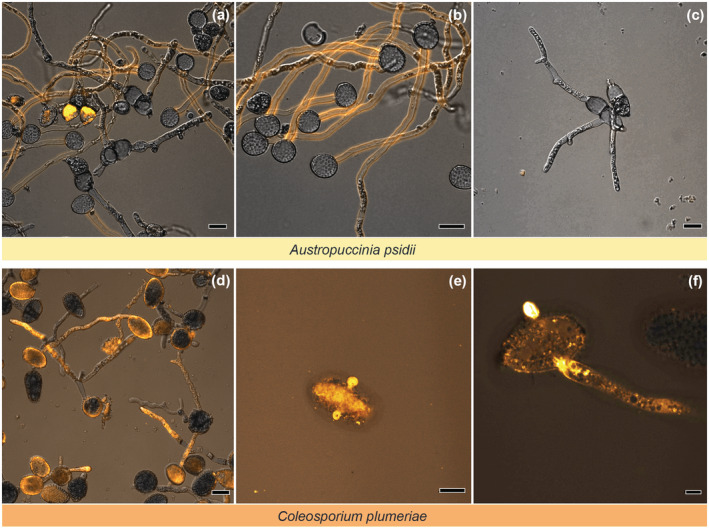
Double‐stranded RNA (dsRNA) either associates externally or is taken up by Austropuccinia psidii and Coleosporium plumeriae urediniospores but not teliospores. Spores were incubated with 1 μg of Cy3‐labelled green fluorescent protein dsRNA on 1% water agar for 6 h (d) or 8 h (a–c,e,f) and were then treated with 75 U of micrococcal nuclease and Cy3 fluorescence observed using a Zeiss LSM700 confocal microscope. A. psidii urediniospores and teliospores are shown before (a) and after (b, c) micrococcal nuclease treatment. (a) A mixture of A. psidii urediniospores and teliospores, (b) A. psidii urediniospores and (c) A. psidii teliospores. C. plumeriae urediniospores at different stages of germination are shown after micrococcal nuclease treatment (d–f). Scale bars represent 20 μm (a–d), 5 μm (e), and 20 μm (f), respectively.
2.2. A. psidii‐specific dsRNA decreases infection on detached leaves
We used detached leaves of Syzygium jambos to examine the impact of dsRNAs that targeted 10 different A. psidii genes: β‐tubulin (β‐TUB), translation elongation factor 1ɑ (EF1‐a), acetyl‐CoA transferase (ATC), cytochrome P450 (CYP450), mitogen activated protein kinase (MAPK), glycine cleavage system H (GCS‐H), 28S ribosomal RNA, and three haustorial targets (HAUS01136, HAUS01215, and HAUS12890) (Figure 2a,b). These genes were chosen based on their broad spectrum of roles as predicted essential genes, genes used successfully in past studies of RNAi, or targets expressed by haustoria (Geiser et al., 2004; Kurtzman & Robnett, 1998; Seifert et al., 2007; Yin et al., 2015). GFP dsRNA was included as a nonspecific control. The number of pustules per leaf decreased significantly in eight of our treatments: EF1‐a, 28S rRNA‐1, β‐TUB, MAPK, ATC, GCS‐H, CYP450, and H01215 (Figure 2a,b,d). There was no difference in pustules per leaf between the –dsRNA control and the nonspecific control (Figure 2c).
FIGURE 2.
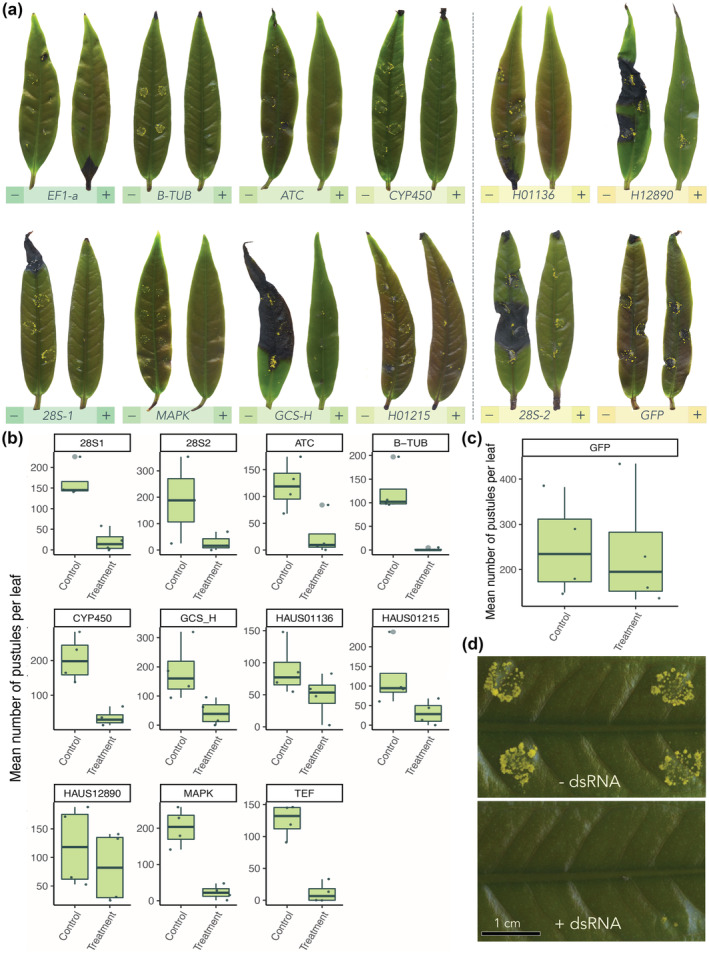
Exogenous application of Austropuccinia psidii‐specific double‐stranded RNA (dsRNA) decreases the incidence of myrtle rust (MR) on Syzygium jambos in detached‐leaf assays. S. jambos leaves (n = 4) infected with A. psidii and treated with 11 A. psidii‐specific dsRNAs were assessed 2 weeks postinoculation. Treatments consisted of 106 A. psidii spores/ml in sterile distilled water containing 0.05% Tween 20 mixed with 100 ng/μl dsRNA. −dsRNA (control) was A. psidii spores in sterile distilled water and 0.05% Tween 20. Green fluorescent protein (GFP) dsRNA was included as a nonspecific control. (a) Boxplot with superimposed scatter representing the mean number of A. psidii pustules per S. jambos leaf (n = 3) treated with 11 A. psidii‐specific dsRNAs. Significance as compared to the control (−dsRNA) is represented by grey asterisks (*p < 0.05, **p < 0.01, ***p < 0.001; Student's t test). This figure was made in R v. 4.0.3 (R Core Team, 2020). (b) Photo comparisons of all dsRNA treatments and controls on S. jambos leaves. For each gene target a control (−) and treatment (+) leaf is shown. Each treatment leaf had a respective control leaf from the sample plant and of the same age, size, and growth stage to ensure accurate comparison. Colours from green to orange represent most to least significant reduction in spore number from control to treatment, respectively. All treatments to the left of the dotted line (ribosomal RNA 28S‐1 (28S‐1), β‐tubulin (β‐TUB), translation elongation factor 1‐α (EF1‐a), mitogen activated protein kinase (MAPK), acetyl CoA‐transferase (ATC), glycine cleavage system‐H (GCS‐H), cytochrome P450 (CYP450) and haustorial target (H01215)) resulted in a significant reduction in pustule number compared to control leaves. All treatments to the right of the dotted line (haustorial target H01136, ribosomal RNA 28S‐2 (28S‐2) and haustorial target H12890) did not result in a significant reduction in pustule number from control to treatment leaves. (c) Boxplot with superimposed scatter representing GFP (nonspecific control) mean number of A. psidii pustules per S. jambos leaf (n = 3) treated with 11 A. psidii‐specific dsRNAs. Significance as compared to the control (−dsRNA) is represented by grey asterisks (*p < 0.05, **p < 0.01, ***p < 0.001; Student's t test). This figure was made in R v. 4.0.3 (R Core Team, 2020). (d) Close‐up photo comparison of control (A. psidii only) and treatment (A. psidii + β‐TUB dsRNA) on S. jambos leaves, taken 2 weeks postinoculation.
Predicted essential genes were more effective targets than genes up‐regulated in the haustoria (H01215, H01136, H12890). Two separate dsRNAs were designed to target the 28S rRNA ribosomal large subunit region. The overlapping 28S rRNA‐1 and 28S rRNA‐2 dsRNA were 340 bp and 685 bp in length, respectively, and we observed a significant inhibition of A. psidii germination in 28S rRNA‐1 treatments compared to 28S rRNA‐2 treatments (Figure 2). This observation was in line with Höfle et al. (2020), who found that efficiency of inducing RNAi decreased with increasing dsRNA length.
2.3. dsRNA inhibits urediniospore germination and formation of appressoria in vitro
We used polyvinylpyrrolidone (PVP)‐treated polydimethylsiloxane (PDMS) discs as artificial leaf‐like surfaces and the three most effective targets from the detached leaf assay, β‐TUB, EF1‐a, and 28S rRNA‐1, to determine the effect of dsRNA treatment on urediniospores of A. psidii and C. plumeriae (Figure 3). The germination rate of A. psidii urediniospores on PDMS decreased significantly when treated with dsRNA that targeted β‐TUB (43% germination), EF1‐a (23% germination), and 28S rRNA‐1 (9% germination) compared to the nonspecific dsRNA (GFP) and ‐dsRNA controls (75% and 78% germination, respectively) (Figure 3a). Germ tubes formed appressoria and infection pegs, with visible cytoplasmic streaming in the GFP and dsRNA controls. Germ tubes of β‐TUB or EF1‐A dsRNA‐treated urediniospores were withered, lacked cytoplasmic streaming, and did not form appressoria and infection pegs. The germination rate was significantly reduced in urediniospores treated with 28S rRNA‐1 dsRNA, and germ tubes were short, straight, and did not produce appressoria (Figure 3c–f).
FIGURE 3.
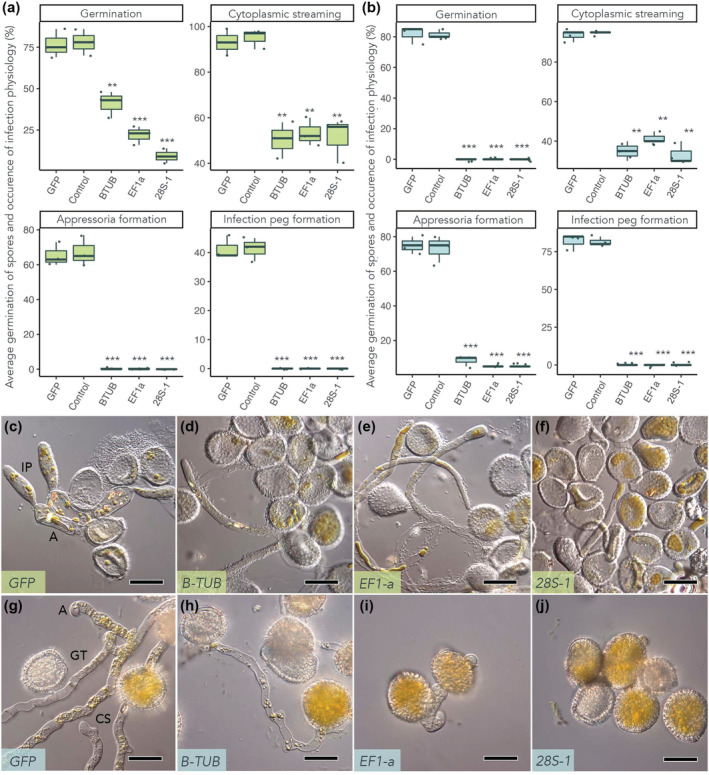
Double‐stranded RNA (dsRNA) targeting essential rust fungi genes significantly alters growth of Austropuccinia psidii and Coleosporium plumeriae urediniospores on polyvinylpyrrolidone (PVP)‐treated polydimethylsiloxane (PDMS) discs. Substrates were inoculated with A. psidii or C. plumeriae and simultaneously treated with β‐tubulin (β‐TUB), translation elongation factor 1‐α (EF1‐a), or ribosomal 28S dsRNA. Treatments contained 106 A. psidii urediniospores/ml mixed with 1 μg/10 μl of pathogen‐specific dsRNA. Control (–dsRNA) is A. psidii or C. plumeriae urediniospores in 0.05% Tween 20. Green fluorescent protein (GFP) dsRNA was included as a nonspecific control. (a, b) Boxplots with scatter representing spore germination rate (n = 3 biological replicates) (as % germination), presence of cytoplasmic streaming, and appressoria and infection peg formation (as % occurrence in germinated spores) in (a) A. psidii and (b) C. plumeriae urediniospores. Significance (as compared to the −dsRNA control group) is represented by asterisks (*p < 0.05, **p < 0.01, ***p < 0.001; Student's t test). One hundred urediniospores were counted per biological replicate and counts were repeated three times on separate substrates. This figure was made in R v. 4.0.3 (R Core Team, 2020). (c–f) Comparison of A. psidii (green) germ‐tube morphology between (c) GFP control and (d) EF1‐a, (e) β‐TUB, and (f) 28S dsRNA treatment groups at 100× magnification. Scale bars represent 20 μm. (g–j) Comparison of C. plumeriae (blue) germ‐tube morphology between (g) GFP control and (h) EF1‐a, (i) β‐TUB, and (j) 28S dsRNA treatment groups at 100× magnification. Scale bars represent 20 μm. A, appressoria; CS, cytoplasmic streaming; GT, germ tube; IP, infection peg. Photos were taken 24 h postinoculation. As no significant difference between −dsRNA (negative control) and GFP dsRNA (nonspecific control) was observed in either species, only GFP dsRNA‐treated urediniospores are shown.
Urediniospores of C. plumeriae treated with β‐TUB, EF1‐a, and 28S rRNA‐1 dsRNA also had decreased germination rates (Figure 3b) and inhibited development of germ tubes and appressoria (Figure 3g–j). The germination rate of C. plumeriae urediniospores was significantly reduced when treated with dsRNA targeting β‐TUB (8.3% germination), EF1‐a (5% germination), or 28S rRNA‐1 (5% germination) compared to the GFP and –dsRNA controls (75% and 73.3% germination, respectively). For all three targets, germ tubes of urediniospores that germinated when treated with dsRNA lacked cytoplasmic streaming and did not form appressoria and infection pegs (Figure 3g–j). Interestingly, Saito et al. (2022) saw a significant reduction in appressoria formation, but not in urediniospore germination, when targeting chitin synthases in P. pachyrhizi.
We attempted to quantify relative gene expression in several different experiments but had inconsistent results, including for controls. Ultimately these experiments will need to use a dsRNA target that does not inhibit spore germination or virulence, and is expressed in germinating rust spores.
2.4. Impact of exogenous dsRNA is concentration‐dependent
To determine whether the effect of dsRNA was proportional to the amount applied, we tested three concentrations (1 ng/μl, 10 ng/μl, and 100 ng/μl) of GFP, β‐TUB, EF1‐a, and 28S rRNA‐1 dsRNA on the germination rate of A. psidii and C. plumeriae urediniospores (Figure 4). Higher concentrations of pathogen‐specific dsRNAs caused significantly lower rates of urediniospore germination in both A. psidii and C. plumeriae (as compared to the −dsRNA control). Germination rates were significantly lower at dsRNA concentrations of 100 ng/μl, as compared to at 10 and 1 ng/μl, for which only two treatments inhibited germination of A. psidii. We also observed uniform germination rates in our nonspecific control group (GFP dsRNA) across all concentrations and in both rusts.
FIGURE 4.
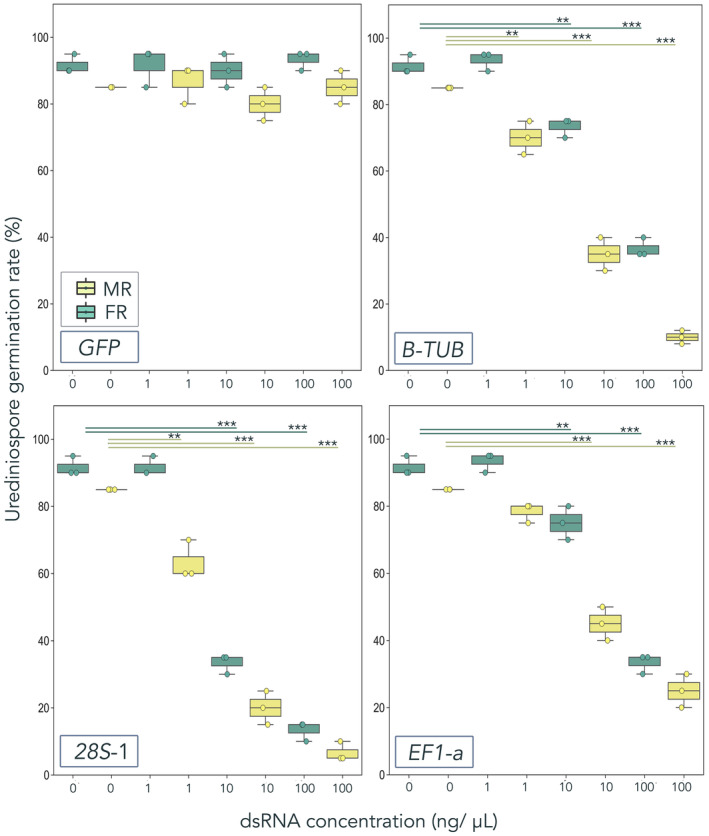
Impacts of exogenously applied dsRNA on Austropuccinia psidii and Coleosporium plumeriae urediniospore germination are concentration‐dependent. Urediniospores were germinated in vitro on polyvinylpyrrolidone (PVP)‐treated polydimethylsiloxane (PDMS) discs. Substrates were inoculated with A. psidii or C. plumeriae and simultaneously treated with β‐tubulin (β‐TUB), translation elongation factor 1‐α (EF1‐a), or ribosomal 28S (28S‐1) dsRNA. Treatments contained 106 A. psidii urediniospores/ml mixed with 100 ng/μl, 10 ng/μl, or 1 ng/μl of pathogen‐specific dsRNA. Boxplots with scatter representing spore germination rate (n = 3 biological replicates) (as % germination) in A. psidii (teal, MR) and C. plumeriae (light green, FR). Significance (as compared to the 0 ng/μl control group) is represented by asterisks (*p < 0.05, **p < 0.01, ***p < 0.001; Student's t test). One hundred urediniospores were counted per biological replicate and counts were repeated three times on separate substrates.
2.5. A. psidii‐specific dsRNA decreases infection in planta
We tested whether exogenous dsRNA could prevent infection of A. psidii on whole S. jambos plants (Figures 5 and S3). Disease incidence (based on the mean diseased portion of new leaves [%]) decreased significantly when inoculum was treated with dsRNA targeting EF1‐a (1.3%) (Figure 4d), β‐TUB (0%) (Figure 4e), or 28S rRNA‐1 (4.7%) (Figure 4f) compared to the −dsRNA (Figure 5b) and GFP (Figure 5c) controls (27% and 25.1%, respectively). dsRNA was stable on S. jambos leaves for at least 1 week after application (Figure 6).
FIGURE 5.
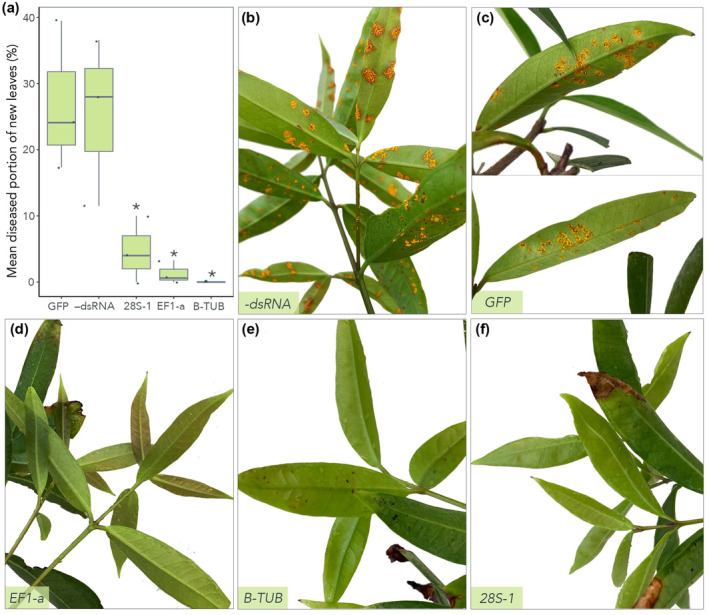
Exogenous application of Austropuccinia psidii‐specific double‐stranded RNA (dsRNA) decreases incidence of myrtle rust (MR) on Syzygium jambos in whole‐plant assays. S. jambos plants were infected with A. psidii and spray treated with three A. psidii‐specific dsRNAs. Treatments contained 106 A. psidii urediniospores/ml mixed with 1 μg/10 μl of β‐tubulin (β‐TUB), translation elongation factor 1‐α (EF1‐a), or ribosomal 28S RNA (28S‐1) dsRNA. Control (−dsRNA) is A. psidii urediniospores in 0.05% Tween 20. Green fluorescent protein (GFP) dsRNA was included as a nonspecific control. (a) Bar plot of diseased tissue as mean (n = 3 biological replicates) percentage coverage of leaf. Each biological replicate represents a separate plant and includes all young (susceptible) growth in the disease assessment (usually four to eight leaves). Bars represent standard error of the mean. Significance (as compared to the −dsRNA control group) is represented by asterisks (*p < 0.05, **p < 0.01, ***p < 0.001; Student's t test). Disease area was measured using the Leaf Doctor application (Pethybridge & Nelson, 2015). This figure was made in R v. 4.0.3 (R Core Team, 2020). (b–f) Photo comparison of S. jambos plants inoculated with A. psidii ((b) −dsRNA control) and treated with (c) GFP, (d) EF1‐a, (e) β‐TUB, or (f) 28S dsRNA. Plants were photographed 2 weeks postinoculation.
FIGURE 6.
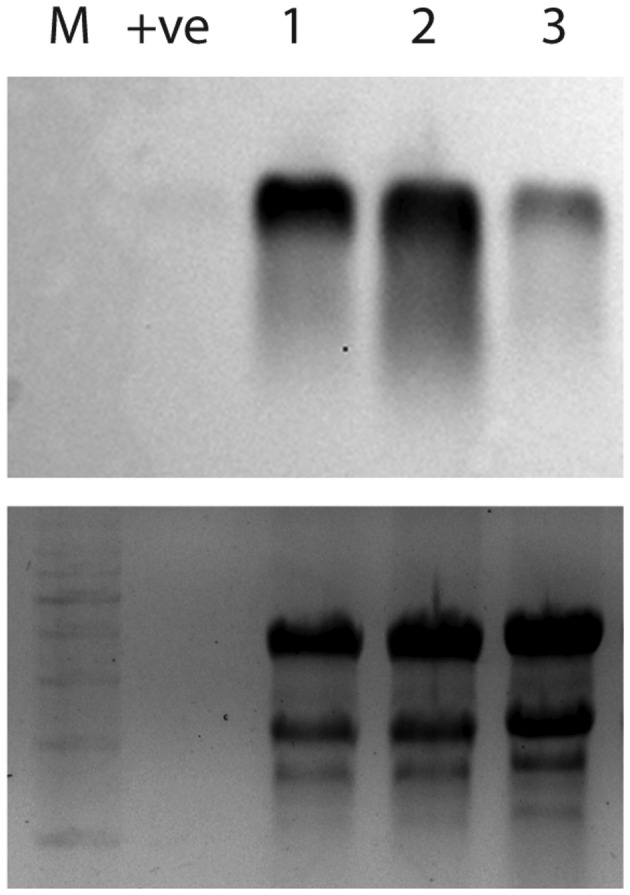
Northern blot showing dsRNA stability on Syzygium jambos leaves 1 week after application. GFP dsRNA (20–40 μl of 250 ng/μl) (Genolution Inc.) in 0.01% Pulse penetrant (Nufarm Australia) was pipetted onto the upper surface of S. jambos leaves (n = 3). Full‐length GFP dsRNA was detected using a digoxygenin‐labelled GFP DNA probe and chemiluminescent detection. 1 ng of GFP dsRNA was run as a positive control (+ve). Upper image is the blot, lower image is ribosomal RNA bands on the gel with 1 kb+ DNA ladder (M) before transfer (loading control).
2.6. RNAi machinery is conserved across rust fungi
We examined conservation of genes in the RNAi pathway for 15 species in eight families of rust fungi to predict whether they may be amenable to control with exogenous RNAi. We found at least one homologue each of Argonaute (AGO), Dicer‐like (DCL), and RNA‐dependent RNA polymerase (RdRP) genes in each species (Figure 7). We did not find evidence of orthology of RNAi pathway genes of the Pucciniomycotina (Basidiomycota) with those identified in Fusarium graminearum, Neurospora crassa (Ascomycota), or Cryptococcus neoformans var. neoformans (Agaricomycotina, Basidiomycota) based on a phylogenetic hypothesis. Most of the examined rust fungi had one homologue of DCL, with the exception of duplicated, orthologous copies or alleles in A. psidii, C. plumeriae, and Skierka agallochae (Figure 7a). Two paralogs of RdRP were recovered in four species of Puccinia and C. plumeriae (Figure 7b), and Araucariomyces fragiformis and A. psidii had either duplicated orthologous copies or two alleles in a dikaryotic genome. All species of rust fungi contained two paralogs of AGO, except A. fragiformis and Hemileia vastatrix, which had one copy each (Figure 7c).
FIGURE 7.
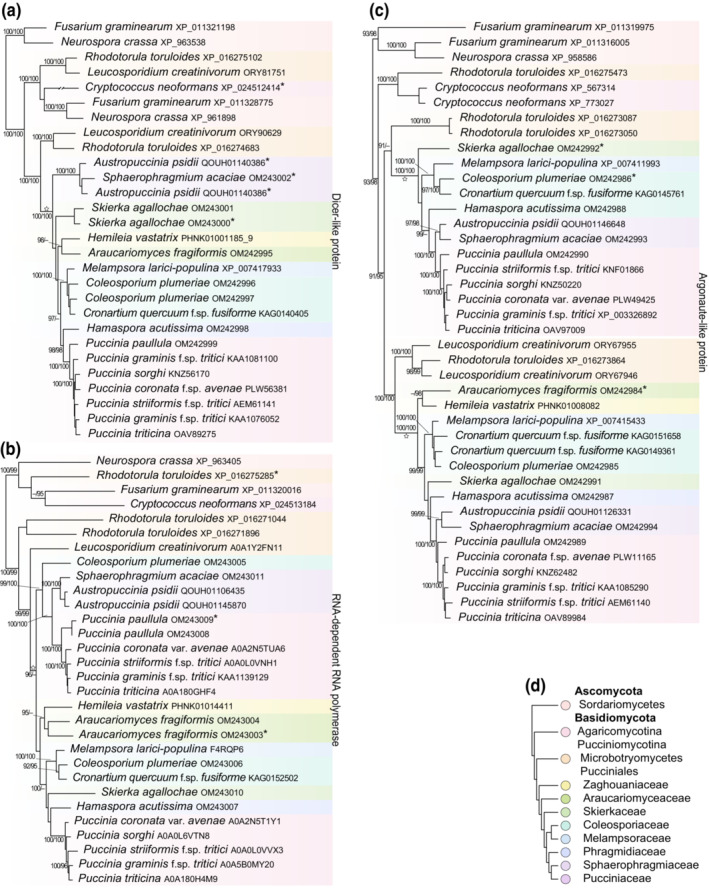
Phylograms of RNAi machinery genes annotated from model fungi and Pucciniomycotina. Support above nodes from 10,000 approximate likelihood ratio tests (≥90) and ultrafast bootstraps (≥95) in IQTree v. 2.0.6. Homologues of (a) Dicer‐like, (b) RNA‐dependent RNA polymerase, and (c) Argonaute‐like genes. *Denotes truncated proteins in an alignment. Taxa are coloured by their phylum, subphylum, class or familial rank classification shown in (d) and based on Aime and McTaggart (2021). Monophyletic groups of rust fungi are marked with a star at their most recent common ancestor.
3. DISCUSSION
Exogenous dsRNA treatment is a potential management solution for rust fungi, and we aimed to determine its utility across the Pucciniales and its mode of action following application of dsRNA on the leaf surface. RNAi has broad potential to manage rust fungi as genes in the RNAi pathway are conserved across the order with evidence of activity in four families. We showed that dsRNA either associates externally or is internalized by germinating urediniospores of rust fungi. dsRNA targeting essential genes inhibited urediniospore germination and appressorium formation on PDMS artificial leaf‐like surfaces and also significantly reduced pustule number on detached leaves and whole plants.
We used two species of rust fungi, A. psidii and C. plumeriae, and demonstrated that exogenous dsRNA prevented urediniospore germination and formation of infection structures. Exogenous application of dsRNA targeting genes with a range of functions (EF1‐a, 28S rRNA‐1, β‐TUB, MAPK, ATC, GCS‐H, CYP450, and H01215) significantly reduced pustule development of A. psidii on detached S. jambos leaves. Urediniospore germination was reduced within 24 h after inoculation on PDMS and treatment with dsRNA that targeted the essential protein‐coding genes β‐TUB, EF1‐a, and 28S rRNA‐1. Urediniospores that germinated had withered germ tubes, lacked cytoplasmic streaming, and did not produce appressoria. This supports our hypothesis that unprocessed dsRNA on the leaf surface can be taken up by the fungus and prevent urediniospore germination and infection.
As biotrophs, in vitro assays are challenging for rust fungi; our urediniospore germination experiments established that PVP‐treated PDMS discs are suited to investigation of the potential of exogenous dsRNA for pathogen control. This innovation of PDMS discs to determine the impact of dsRNA on a leaf‐like surface builds on the use of artificial surfaces in earlier studies of spore germination and formation of appressoria (Bronkhorst et al., 2021; Collins, 1996; Saito et al., 2022; Stone et al., 2012). Our study is the first to use these PDMS leaf‐like surfaces to visualize the impact of dsRNA on the formation of infection structures, such as appressoria and infection pegs, in rust fungi.
The most effective inhibition of A. psidii and C. plumeriae urediniospore germination was observed at dsRNA concentrations of 100 ng/μl. The significant decrease in spore germination with increased dsRNA concentration suggests that the optimal working concentration is higher than in other RNAi studies that have used a concentration of 20 ng/μl (Hu et al., 2020; Koch et al., 2016; Ruiz‐Jiménez et al., 2021). Based on the successful inhibition of disease in our assays, the ideal dsRNA concentration (to maximize impact and minimize cost) is closer to 100 ng/μl, which we plan to optimize for applied uses in agriculture and conservation.
Exogenously applied dsRNA prevented or significantly reduced infection by A. psidii on whole plants, and highlights its potential to manage rust fungi in environmental or agricultural systems. Combined with our finding that dsRNA either associates externally or is internalized by germinating urediniospores, exogenous dsRNA treatment may be used to manage outbreaks of rust fungi caused by these clonal spores. A limitation of disease management using this approach is our finding that dsRNA is not internalized by teliospores and therefore may not prevent meiosis and overwintering, although this finding requires testing in teliospores of additional taxa. Urediniospores of rust fungi germinate through germ pores, which may provide a point of entry and an explanation for the difference between association of dsRNA with urediniospores and teliospores. Sclerotinia sclerotiorum takes up dsRNA via clathrin‐mediated endocytosis (Wytinck et al., 2020) and this mechanism may resolve whether dsRNA is actively taken up by germinating rust spores. Knowledge of whether basidiospores, produced by meiosis, take up dsRNA may help coordinate dsRNA application and management of sexual reproduction in life/disease cycles, with the potential to prevent outbreaks of new strains of rust fungi.
Genes encoding the RNAi pathway are conserved in eight families of rust fungi examined in the present study, and Hu et al. (2020) demonstrated activity of this pathway in a ninth family, Phakopsoraceae (P. pachyrhizi). Based on conservation of genes in the RNAi pathway across taxa in the Pucciniales, we hypothesize that RNAi could be used to manage all species of rust fungi.
Treatment with exogenous dsRNA targeting essential genes is a potential management option for rust fungi beyond the studied species, as dsRNA can be readily made using universal primers without knowledge of the gene sequence in target taxa. Off‐target effects outside of Pucciniales are unlikely based on the variability of these genes as molecular barcodes, short target sequence length, and high degree of sequence homology required for silencing (Seifert et al., 2007). Our findings challenge hypotheses that highly transcribed targets are less suited to RNAi (Larsson et al., 2010; McLoughlin et al., 2018) and support similar findings in Fusarium sp., Botrytis cinerea, Magnaporthe oryzae, and Colletotrichum truncatum, where RNAi against β‐tubulin and translation elongation factor 2 inhibited fungal growth, deformed mycelia, and reduced development of infection structures and conidiation (Gu et al., 2019; Mosa & Youssef, 2021; Nerva et al., 2020). Our finding that dsRNAs targeting highly transcribed genes significantly reduce urediniospore germination and disease symptoms may suggest that the RNAi mechanism is amplified in rust fungi, in contrast to previous reports for other fungi (Song et al., 2018). The hypothesis that RdRPs detected in our phylogenetic analysis enhance RNAi in rust fungi requires further testing.
RNAi has an advantage over fungicide application and breeding for resistance, for which point mutations in the fungus and outcrossing of plants may overcome disease resistance (McDonald et al., 2019). To develop resistance to sprays containing long dsRNA as the active ingredient, entire targeted genes or RNAi genes must be lost or accumulate mutations at multiple sites in a targeted region to avoid recognition of the corresponding mRNA by siRNAs. New pathogen target sequences can be identified more rapidly than breeding layers of resistance into host plants, and RNAi has fewer environmental impacts than chemical fungicides. Delivery, uptake, and stability of exogenously applied dsRNA are improved when complexed with RNA carriers (Islam et al., 2021; Leber et al., 2017; Mitter et al., 2017; Numata et al., 2014), and with further developments in longevity of dsRNA on the leaf surface, an outcome of our study is a potential disease management strategy to apply in fragile ecosystems or on culturally sacred trees threatened by myrtle rust.
Rust fungi have the largest genomes of all known fungi (Tavares et al., 2014). The six new genomes assembled in the present study were over 500 Mb, larger than the average genome size of rust fungi (300 Mb), with the exception of C. plumeriae (c.187 Mb) and Sphaerophragmium acaciae (c.244 Mb). These genomes are the first sequenced and assembled for taxa in the Araucariomyceaceae, Phragmidiaceae, and Skierkaceae. The gene tree relationships among conserved RNAi machinery were mostly congruent with the familial relationships recovered by Aime and McTaggart (2021).
Exogenous dsRNA treatment brings a new era for disease management of rust fungi. It probably has order‐wide potential based on conservation of genes in the studied taxa. It can be timed with disease outbreaks similar to fungicides, but without negative environmental effects. It has advantages over breeding resistance and fungicide application in that resistance to dsRNA requires loss of essential genes, or loss of the silencing pathway, and our study shows its potential for the management of invasive rust fungi, such as A. psidii, in natural environments. We have shown that exogenous application of dsRNA prevents infection by rust fungi, and a future challenge is whether this approach can be used curatively to manage established infections.
4. EXPERIMENTAL PROCEDURES
4.1. Preparation of inocula
Urediniospores of A. psidii were shaken from infected S. jambos plants grown under shade‐house conditions. Urediniospores of C. plumeriae were vacuumed from infected leaves. For all experiments except dsRNA uptake, spores were dried for 48 h in a desiccator then aliquoted and stored at −80°C. Urediniospores were rehydrated in sterile distilled water and 0.05% Tween 20 for 30 min before use. Inocula were concentrated to 106 spores/ml. Urediniospores of A. psidii and C. plumeriae were germinated at 16 and 26°C, respectively, with 80% relative humidity. These conditions were used for whole‐plant inoculations and spore germination experiments.
4.2. RNA extraction and dsRNA synthesis
Dried urediniospores of A. psidii and C. plumeriae were ground in liquid nitrogen and total RNA extracted using TRIzol (ThermoFisher Scientific) as per the manufacturer's instructions. cDNA was synthesized from extracted RNA using the ProtoScript II First Strand cDNA Synthesis Kit, following the standard protocol (New England BioLabs). Target sequences (300–400 bp long) were amplified from cDNA using sequence‐specific primers containing T7 promoter sequences at the 5′ end (Table S1). Contigs that contained target genes in assemblies of A. psidii and C. plumeriae were identified by tBLASTn, and exons were predicted in UniProt.
dsRNA was synthesized in vitro with a HiScribe T7 High Yield RNA Synthesis Kit (New England Biolabs) using the T7 PCR products as template. Sense and antisense sequences were synthesized in the same reaction. Following dsRNA synthesis, RNA was DNase‐treated and purified using TRIzol and annealed at 95°C for 5 min. For Cy3 dsRNA synthesis, 4.5 μl of 5 mM Cy3 CTP (GE Healthcare) and 1.125 μl of 100 mM CTP were used instead of 1.5 μl of 100 mM CTP nucleotides. RNA was purified using the Monarch RNA Cleanup Kit (New England BioLabs).
4.3. dsRNA uptake assays
An aliquot of 4 μl of 250 ng/μl Cy3‐labelled GFP dsRNA was mixed with 4 μl of fresh rust spores (104–106 spores/ml) and plated on 1% water agar drops on microscope slides or directly on the microscope slides, according to the method described by Odell and Smith (1982). Slides were placed on moist filter paper in a Petri dish sealed with Parafilm. Spores were incubated for 6 or 8 h in the dark at room temperature, treated with 75 U of micrococcal nuclease (New England BioLabs) for 30 min at 37°C, and examined under a Zeiss LSM700 confocal microscope for Cy3 fluorescence (excitation at 555 nm and detection at 559–640 nm).
4.4. Fluorescence lifetime imaging microscopy
Spores were set up directly on glass slides as detailed above but with 0.5 μg of dsRNA/8 μl drop instead of 1 μg. Spores were incubated in the dark at room temperature for 4 h before treating with micrococcal nuclease. Imaging was performed on a Leica Microsystems SP8 system, with Cy3 excited using a 40 MHz pulsed, tunable white light laser at 555 nm and detected with HyD detectors at 560–620 nm. FLIM was performed to distinguish the autofluorescence from the spore and the Cy3 fluorescence signal. Data were collected to obtain a minimum of 100 photons per pixel to ensure reliable modelling of the fluorescence decay. Leica Application Suite X (LAS X) software v. 5.0.2 (https://imb.uq.edu.au/facilities/microscopy/microscopy‐hardware‐software) was used to produce FLIM images and phasor plots. ImageJ v. 1.53q was used for processing images and Z‐stack projections.
4.5. Detached leaf assays
Young, emerging S. jambos leaves were excised at the base of the petiole using a scalpel. In a laminar flow, leaves were surface sterilized in 1% sodium hypochlorite for 1 min and washed for 3 × 1 min in Milli‐Q water. Then, 1% agar was pipetted along one side of sterile Petri dishes, approximately 3 ml, covering a quarter of the Petri dish. Leaves were transferred onto the Petri dishes using sterile forceps, with the petiole penetrating the medium. Inoculum (106 spores/ml in 0.05% Tween 20) was pipetted onto leaves in 5‐μl droplets, with two to 10 droplets per leaf, depending on leaf size. Inoculum was resuspended by inverting every 30 s to prevent settling of spores. Plates were sealed using Parafilm and left in the dark for 24 h at 18°C, 80% humidity. After 24 h, plates were moved to an incubator (25°C, 16 h day, 8 h night cycle) for 14 days.
For assays including dsRNA, aliquots of A. psidii inoculum were prepared in PCR tubes from a 106 spores/ml stock solution and 1 μg of dsRNA was added per 10 μl of A. psidii inoculum. Each plate contained one control (−dsRNA) and one treatment leaf (+dsRNA), with the same number of drops on each leaf. Control and treatment leaves in each plate were from the same S. jambos plant and were of the same age and growth stage to ensure minimal variation in cuticle thickness and other infection‐impacting features.
4.6. Urediniospore germination assays on PDMS discs
Ten microlitres of A. psidii or C. plumeriae inocula were mixed with 1 μg of GFP, EF1‐a, 28S rRNA‐1 or β‐TUB dsRNA, with 0.05% Tween 20 as the negative control, and pipetted in 2 μl aliquots onto discs. Urediniospores were incubated for 24 h at 18°C, 80% humidity, then photographed using a Leica microscope with Nomarski interference and a DFC550 camera. Germinated urediniospores were counted using Adobe Photoshop v. 22.1.0.
4.7. dsRNA dose‐dependent assays
Urediniospores were germinated on discs made of polyvinylpyrrolidone (PVP, Kollidon 25; Sigma‐Aldrich)‐treated PDMS (Sylgard 184, 10:1 wt/wt base to curing agent ratio) (Soffe et al., 2019). A standard PDMS microfabrication processes produced discs 2 mm high, and subsequent PVP treatment made surfaces hydrophilic to facilitate wetting with spore germination buffer. PDMS discs were placed in Petri dishes on damp filter paper.
Purified GFP (nonspecific control), EF1‐a, β‐TUB, and 28S rRNA‐1 dsRNA were diluted to three concentrations: 100 ng/μl, 10 ng/μl, and 1 ng/μl. dsRNAs were mixed with either A. psidii or C. plumeriae urediniospores at 106 spores/ml and 10 μl of this solution was aliquoted in 2 μl droplets onto PVP‐coated PDMS discs. Inoculum only (−dsRNA) was also included as a negative control. Urediniospores were incubated for 24 h at 18°C, 80% humidity and germinated spores were quantified using a Leica microscope.
4.8. Shade‐house assays
One‐year‐old cuttings of S. jambos plants were grown in a shade‐house, watered twice daily for 5 min with an overhead sprinkler system and fertilized with Nitrosol 2 weeks prior to infection to encourage growth of new leaves. A. psidii inoculum was aliquoted into 1 ml fine‐spray bottles with 100 ng/μl of dsRNA for each treatment. Young flush was sprayed until saturated (c.1 ml). Plants were covered and incubated in the dark for 24 h at 18°C, 80% humidity. Coverings were removed and plants were returned to the shade‐house (ambient temperature, natural light, and overhead watering for 2 × 5 min daily). Infections were monitored and final disease incidence was assessed 2 weeks postinoculation. Disease was scored based on manual measurements of lesion size and percentage coverage of diseased tissue using the Leaf Doctor application (Pethybridge & Nelson, 2015).
4.9. Northern blots
Commercially produced GFP dsRNA (Genolution Inc.) was pipetted onto the upper surface of S. jambos leaves. 20–40 μl of 250 ng/μl dsRNA in 0.01% Pulse penetrant (Nufarm Australia) was applied per leaf depending on the size. dsRNA was applied slowly and spread across the leaves via pipetting to promote even spread of dsRNA. Leaves were harvested 1 week later and RNA was extracted using the Norgen Plant/Fungi Total RNA Purification Kit following the manufacturer's protocol (Norgen Biotek Corp.). Extracted RNA was run on a 1% agarose formaldehyde gel (10 μl/lane) and then transferred onto a Hybond‐N+ nylon membrane (GE Healthcare). The blot was probed with a digoxigenin (DIG)‐labelled GFP DNA probe (Roche), which was detected using an anti‐digoxigenin AP‐conjugate (Roche) and visualized using the CDP Star chemiluminescent substrate (Sigma Aldrich) on an iBright imaging system (Thermo Fisher).
4.10. Genome sequencing and assembly
We sequenced and assembled draft genomes of A. fragiformis (Araucariomyceaceae), C. plumeriae (Coleosporiaceae), Hamaspora acutissima (Phragmidiaceae), Puccinia paullula (Pucciniaceae), S. agallochae (Skierkaceae), and S. acaciae (Sphaerophragmiaceae). Genomic DNA was extracted from environmental samples of rust fungi (Table 1). Urediniospores were vacuumed directly from leaf material, ground in liquid nitrogen, and DNA was extracted following the protocol of a Machery Nagel NucleoSpin Plant II DNA extraction kit. The Australian Genome Research Facility (AGRF) prepared a Chromium 10× library and sequenced all libraries on an Illumina NovaSeq 6000. Chromium sequencing data were assembled using Supernova v. 2.1.1 (Weisenfeld et al., 2017).
TABLE 1.
Assembly statistics of genomes assembled in the present study
| Taxon | Specimen number | Family | DNA (ng) for library preparation | No. of reads (millions) | Assembly size (Mb) scaffolds >10 kb | Effective coverage (%) | Repetitive fraction (%) | GC content (%) | N50 (kb) |
|---|---|---|---|---|---|---|---|---|---|
| Araucariomyces fragiformis | BRIP 68996 | Araucariomyceaceae | 3016 | 890.11 | 622.65 | 20.49 | 28.73 | 51.97 | 33.4 |
| Coleosporium plumeriae | BRIP 69006 | Coleosporiaceae | 239 | 822.42 | 186.92 | 18.73 | 92.65 | 43.19 | 22.6 |
| Hamaspora acutissima | BRIP 69009 | Phragmidiaceae | 1462 | 965.77 | 708.12 | 21.87 | 70.36 | 53.45 | 52.0 |
| Puccinia paullula | BRIP 69007 | Pucciniaceae | 2105 | 925.67 | 941.82 | 23.16 | 41.89 | 46.15 | 40.3 |
| Skierka agallochae | BRIP 69008 | Skierkaceae | 2400 | 909.86 | 1060 | 30.39 | 27.39 | 33.66 | 26.5 |
| Sphaerophragmium acaciae | BRIP 69010 | Sphaerophragmiaceae | 3360 | 881.55 | 244.19 | 19.4 | 76.26 | 42.16 | 20.2 |
4.11. RNAi gene discovery
Published genomes of A. psidii (McTaggart et al., 2018), Cronartium quercuum f. sp. fusiforme (Coleosporiaceae) (Pendleton et al., 2014), H. vastatrix (Zaghouaniaceae) (Cristancho et al., 2014), Melampsora larici‐populina (Melampsoraceae) (Duplessis et al., 2011), Puccinia coronata f. sp. avenae (Pucciniaceae) (Miller et al., 2018), Puccinia graminis f. sp. tritici (Duplessis et al., 2011), Puccinia sorghi (Rochi et al., 2018), Puccinia striiformis f. sp. tritici (Cuomo et al., 2017), and Puccinia triticina (Kiran et al., 2016) were mined for copies of RNAi genes (DCLs, RdRPs and AGOs) using tBLASTn (Altschul et al., 1990). We searched for homologues of genes in the RNAi pathway in C. neoformans (Janbon et al., 2010), F. graminearum (Chen et al., 2015), and Cryphonectria parasitica (Zhang et al., 2014). Coding sequences of potential homologues were predicted using comparative models of P. graminis, P. sorghi, and M. larici‐populina on UniProt (Bateman et al., 2020). Predicted coding sequences were aligned with 100 HMM iterations in Clustal Omega using Ugene (Okonechnikov et al., 2012). The most likely tree was searched for from each alignment using IQTree v. 2.0.6 with the best fit model of evolution (command‐m TEST), and 10,000 ultrafast bootstraps and approximate likelihood ratio tests (Hoang et al., 2018; Nguyen et al., 2015).
4.12. Data analysis and visualization
Student's t tests were computed in R v. 4.0.3 (R Core Team, 2020) using the t‐test function and the dplyr package (Wickham et al., 2020). p values of <0.05 were considered significant. Data were plotted in R v. 4.0.3 (R Core Team, 2020) using bar (geom_bar) and box (geom_box) plots with the ggplot2 package (Wickham et al., 2020).
CONFLICT OF INTEREST
The authors declare no competing financial and nonfinancial interests.
ACCESSION NUMBERS
Afragiforme_AGO_620928, OM242984; Coleosporium_AGO_674944, OM242985; Coleosporium_AGO_675696, OM242986; Hamaspora_AGO1_977380, OM242987; Hamaspora_AGO2_613271, OM242988; PucciniaP_AGO_894603, OM242989; PucciniaP_AGO_941392, OM242990; Skierka_AGO_935665, OM242991; Skierka_AGO_990216,OM242992; Sphaerophragmium_AGO2_1035541, OM242993; Sphaerophragmium_AGO_6394, OM242994; Afragiforme_DCL_1423528, OM242995; Coleosporium_DCL1_4378, OM242996; Coleosporium_DCL_651311, OM242997; Hamaspora_DCL1_931648, OM242998; PucciniaP_DCL_897422, OM242999; Skierka_DCL_951268, OM243000; Skierka_DCL_984643, OM243001; Sphaerophragmium_DCL1_94389, OM243002; Afragiforme_RDRP_1026684, OM243003; Afragiforme_RDRP_1457563, OM243004; Coleosporium_RDRP_11429, OM243005; Coleosporium_RDRP_686038, OM243006; Hamaspora_RDRP_618127, OM243007; PucciniaP_RDRP_859667, OM243008; PucciniaP_RDRP_906243, OM243009; Skierka_RDRP_950972, OM243010; Sphaerophragmium_RDRP_460606, OM243011.
Supporting information
Figure S1 Coleosporium plumeriae Cy3‐CTP and Cy3‐dsRNA uptake assay. C. plumeriae urediniospores were incubated with 500 ng of Cy3‐labelled GFP dsRNA for 4 h and were then treated with 75 U of micrococcal nuclease. One biological replicate was set up for each treatment. The first image in each row is merged bright field (BF) and Cy3 fluorescence, the second is Cy3 lifetime, and the third is the corresponding phasor plot. Strong Cy3 fluorescence was seen in the germ tubes of spores treated with Cy3‐dsRNA, while only background fluorescence could be seen in the germ tubes of spores treated with Cy3‐CTP (negative control). Note that one nongerminated spore treated with Cy3‐CTP showed a strong fluorescence signal. Fluorescence lifetime imaging microscopy (FLIM, Datta et al., 2020) showed a difference in lifetime of Cy3 incorporated in dsRNA compared to in free CTP nucleotides. FLIM images were taken on a Leica Microsystems SP8 system, scale bars correspond to 20 μm
Figure S2 Selected Cy3 dsRNA_1 Z‐stack confocal images. The complete Z‐stack consisted of 33 images across a depth of 10.65 μm. Images were taken on a Leica Microsystems SP8 system, scale bars correspond to 20 μm
Figure S3 Examples of individual leaf photos used for disease coverage ratings in planta. Control (−dsRNA and GFP dsRNA) leaves both exhibit significant myrtle rust (MR) symptoms. One small MR lesion can be seen on the EF1‐a treatment leaf (yellow pustules to the right of the midvein). No MR symptoms can be seen on the β‐TUB treatment leaf
Table S1 Primers for in vitro synthesis of Austropuccinia psidii (Ap) and Coleosporium plumeriae (Cp)‐specific dsRNA. Transcripts per million (TPM) was calculated from unpublished RNA‐sequencing data
ACKNOWLEDGEMENTS
We thank the Research Computing Centre (RCC) at the University of Queensland for providing computational resources for genomic analyses and Dr Deborah Barkauskas from the Institute for Molecular Bioscience Microscopy facility at the University of Queensland for conducting the FLIM. A.R.M. acknowledges the University of Queensland Development Fellowships (UQFEL1718905) and support from the Department of the Environment and Energy under the Australian Biological Resources Study (grant number RG18‐43). A.S. was supported by an Advance Queensland Industry Research Fellowship. The production and initial assessment of the PDMS discs for A. psidii pathology studies was supported by the New Zealand Government MBIE Beyond Myrtle Rust program (C09X1806). We thank the Australian Plant Biosecurity Foundation for their support and New England BioLabs for providing molecular biology enzymes and kits. We thank Dr Bill Taylor, Kieran Murphy, and Dr Chris Brosnan for their valuable feedback on the manuscript. We thank Steven Fletcher for his coding assistance in the dose‐dependent boxplots. We thank Benjamin Petre, Julie Lintz, and an anonymous reviewer for their suggestions that improved the manuscript.
Degnan, R.M. , McTaggart, A.R. , Shuey, L.S. , Pame, L.J.S. , Smith, G.R. & Gardiner, D.M. et al. (2023) Exogenous double‐stranded RNA inhibits the infection physiology of rust fungi to reduce symptoms in planta. Molecular Plant Pathology, 24, 191–207. Available from: 10.1111/mpp.13286
Rebecca M. Degnan and Alistair R. McTaggart contributed equally to the work.
Contributor Information
Neena Mitter, Email: n.mitter@uq.edu.au.
Anne Sawyer, Email: a.sawyer@uq.edu.au.
DATA AVAILABILITY STATEMENT
Draft assemblies of all genomes sequenced in the present study are available at Zenodo (10.5281/zenodo.6650763) or at https://drive.google.com/drive/folders/1nDRWYLEO80R6CCxdnMzkaFY3DqUKn6Vo?usp=sharing. Raw sequencing data are available on request from ARM. All sequences used for analyses of genes in the RNAi pathway have been deposited in GenBank. Raw germination rate and disease assessment data that support the findings of this study are made available publicly through Dryad (10.5281/zenodo.7018192).
REFERENCES
- Aime, M. & McTaggart, A. (2021) A higher‐rank classification for rust fungi, with notes on genera. Fungal Systematics and Evolution, 7, 21–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida, R.F. , Machado, P.S. , Damacena, M.B. , Santos, S.A. , Guimarães, L.M.S. , Klopfenstein, N.B. et al. (2021) A new, highly aggressive race of Austropuccinia psidii infects a widely planted, myrtle rust‐resistant, eucalypt genotype in Brazil. Forest Pathology, 51, e12679. [Google Scholar]
- Altschul, S.F. , Gish, W. , Miller, W. , Myers, E.W. & Lipman, D.J. (1990) Basic local alignment search tool. Journal of Molecular Biology, 215, 403–410. [DOI] [PubMed] [Google Scholar]
- Babu, P. , Baranwal, D.K. , Pal, D. , Bharti, H. , Joshi, P. , Thiyagarajan, B. et al. (2020) Application of genomics tools in wheat breeding to attain durable rust resistance. Frontiers in Plant Science, 11, 567147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman, A. , Martin, M.‐J. , Orchard, S. , Magrane, M. , Agivetova, R. , Ahmad, S. et al. (2020). UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Research, 49, D480–D489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulcombe, D. (2004) RNA silencing in plants. Nature, 431, 356–363. [DOI] [PubMed] [Google Scholar]
- Bernstein, E.O. & Jones, C.B. (1969) Skin replication procedure for the scanning electron microscope. Science, 166, 252–253. [DOI] [PubMed] [Google Scholar]
- Bologna, N.G. & Voinnet, O. (2014) The diversity, biogenesis, and activities of endogenous silencing small RNAs in Arabidopsis . Annual Reviews in Plant Biology, 65, 473–503. [DOI] [PubMed] [Google Scholar]
- Bronkhorst, J. , Kasteel, M. , Van Veen, S. , Clough, J.M. , Kots, K. , Buijs, J. et al. (2021) A slicing mechanism facilitates host entry by plant‐pathogenic Phytophthora . Nature Microbiology, 6, 1000–1006. [DOI] [PubMed] [Google Scholar]
- Cai, Q. , He, B. , Kogel, K.H. & Jin, H. (2018) Cross‐kingdom RNA trafficking and environmental RNAi—nature's blueprint for modern crop protection strategies. Current Opinion in Microbiology, 46, 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Gao, Q. , Huang, M. , Liu, Y. , Zunyong, L. , Liu, X. et al. (2015) Characterization of RNA silencing components in the plant pathogenic fungus Fusarium graminearum . Scientific Reports, 5, 12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, T.J. (1996) Appressorium induction in the cereal rusts. PhD thesis. Edinburgh, UK: University of Edinburgh. [Google Scholar]
- Collins, T.J. & Read, N.D. (1997) Appressorium induction by topographical signals in six cereal rusts. Physiological and Molecular Plant Pathology, 51, 169–179. [Google Scholar]
- Cristancho, M.A. , Botero‐Rozo, D.O. , Giraldo, W. , Tabima, J. , Riaño‐Pachón, D.M. , Escobar, C. et al. (2014) Annotation of a hybrid partial genome of the coffee rust (Hemileia vastatrix) contributes to the gene repertoire catalog of the Pucciniales. Frontiers in Plant Science, 5, 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuomo, C.A. , Bakkeren, G. , Khalil, H.B. , Panwar, V. , Joly, D. , Linning, R. et al. (2017) Comparative analysis highlights variable genome content of wheat rusts and divergence of the mating loci. G3 (Bethesda), 7, 361–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmay, T. , Hamilton, A. , Rudd, S. , Angell, S. & Baulcombe, D.C. (2000) An RNA‐dependent RNA polymerase gene in Arabidopsis is required for post‐transcriptional gene silencing mediated by a transgene but not by a virus. Cell, 101, 543–553. [DOI] [PubMed] [Google Scholar]
- Datta, R. , Heaster, T.M. , Sharick, J.T. , Gillette, A.A. & Skala, M.C. (2020) Fluorescence lifetime imaging microscopy: fundamentals and advances in instrumentation, analysis, and applications. Journal of Biomedical Optics, 25, 1–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta, S. , Jha, S.K. , Prabhu, K.V. , Kumar, M. & Mukhopadhyay, K. (2019) Leaf rust (Puccinia triticina) mediated RNAi in wheat (Triticum aestivum L.) prompting host susceptibility. Functional & Integrative Genomics, 19, 437–452. [DOI] [PubMed] [Google Scholar]
- Dickinson, S. (1949) Studies in the physiology of obligate parasitism: II. The behaviour of the germ‐tubes of certain rusts in contact with various membranes. Annals of Botany, 13, 219–236. [Google Scholar]
- Doan, H.K. & Leveau, J.H.J. (2015) Artificial surfaces in phyllosphere microbiology. Phytopathology, 105, 1036–1042. [DOI] [PubMed] [Google Scholar]
- Drenth, A. , McTaggart, A.R. & Wingfield, B.D. (2019) Fungal clones win the battle, but recombination wins the war. IMA Fungus, 10, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey, H. , Kiran, K. , Jaswal, R. , Bhardwaj, S.C. , Mondal, T.K. , Jain, N. et al. (2020) Identification and characterization of Dicer‐like genes in leaf rust pathogen (Puccinia triticina) of wheat. Functional & Integrative Genomics, 20, 711–721. [DOI] [PubMed] [Google Scholar]
- Duplessis, S. , Cuomo, C.A. , Lin, Y.C. , Aerts, A. , Tisserant, E. , Veneault‐Fourrey, C. et al. (2011) Obligate biotrophy features unraveled by the genomic analysis of rust fungi. Proceedings of the National Academy of Sciences of the United States of America, 108, 9166–9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, J.G. , Lagudah, E.S. , Spielmeyer, W. & Dodds, P.N. (2014) The past, present and future of breeding rust resistant wheat. Frontiers in Plant Science, 5, 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fensham, R.J. & Radford‐Smith, J. (2021) Unprecedented extinction of tree species by fungal disease. Biological Conservation, 261, 109276. [Google Scholar]
- Fletcher, S.J. , Reeves, P.T. , Hoang, B.T. & Mitter, N. (2020) A perspective on RNAi‐based biopesticides. Frontiers in Plant Science, 11, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser, D.M. , Del Mar Jiménez‐Gasco, M. , Kang, S. , Makalowska, I. , Veeraraghavan, N. , Ward, T.J. et al. (2004) FUSARIUM‐ID v. 1.0: a DNA sequence database for identifying Fusarium . European Journal of Plant Pathology, 110, 473–479. [Google Scholar]
- Gill, U.S. , Sun, L. , Rustgi, S. , Tang, Y. , Wettstein, D. & Mysore, K.S. (2018) Transcriptome‐based analyses of phosphite‐mediated suppression of rust pathogens Puccinia emaculata and Phakopsora pachyrhizi and functional characterization of selected fungal target genes. The Plant Journal, 93, 894–904. [DOI] [PubMed] [Google Scholar]
- Glen, M. , Alfenas, A. , Zauza, E. , Wingfield, M. & Mohammed, C. (2007) Puccinia psidii: a threat to the Australian environment and economy. Australasian Plant Pathology, 36, 1–16. [Google Scholar]
- Gu, K.X. , Song, X.S. , Xiao, X.M. , Duan, X.X. , Wang, J.X. , Duan, Y.B. et al. (2019) A β(2)‐tubulin dsRNA derived from Fusarium asiaticum confers plant resistance to multiple phytopathogens and reduces fungicide resistance. Pesticide Biochemistry and Physiology, 153, 36–46. [DOI] [PubMed] [Google Scholar]
- Höfle, L. , Biedenkopf, D. , Werner, B.T. , Shrestha, A. , Jelonek, L. & Koch, A. (2020) Study on the efficiency of dsRNAs with increasing length in RNA‐based silencing of the Fusarium CYP51 genes. RNA Biology, 17, 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang, D.T. , Chernomor, O. , Von Haeseler, A. , Minh, B.Q. & Vinh, L.S. (2018) UFBoot2: improving the ultrafast bootstrap approximation. Molecular Biology and Evolution, 35, 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holoch, D. & Moazed, D. (2015) RNA‐mediated epigenetic regulation of gene expression. Nature Reviews Genetics, 16, 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, D. , Chen, Z.Y. , Zhang, C. & Ganiger, M. (2020) Reduction of Phakopsora pachyrhizi infection on soybean through host‐ and spray‐induced gene silencing. Molecular Plant Pathology, 21, 794–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam, M.T. , Davis, Z. , Chen, L. , Englaender, J. , Zomorodi, S. , Frank, J. et al. (2021) Minicell‐based fungal RNAi delivery for sustainable crop protection. Microbial Biotechnology, 21, 1847–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janbon, G. , Maeng, S. , Yang, D.H. , Ko, Y.J. , Jung, K.W. , Moyrand, F. et al. (2010) Characterizing the role of RNA silencing components in Cryptococcus neoformans . Fungal Genetics and Biology, 47, 1070–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran, K. , Rawal, H.C. , Dubey, H. , Jaswal, R. , Devanna, B.N. , Gupta, D.K. et al. (2016) Draft genome of the wheat rust pathogen (Puccinia triticina) unravels genome‐wide structural variations during evolution. Genome Biology and Evolution, 8, 2702–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, A. , Biedenkopf, D. , Furch, A. , Weber, L. , Rossbach, O. , Abdellatef, E. et al. (2016) An RNAi‐based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathogens, 12, e1005901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzman, C.P. & Robnett, C.J. (1998) Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Van Leeuwenhoek, 73, 331–371. [DOI] [PubMed] [Google Scholar]
- Larsson, E. , Sander, C. & Marks, D. (2010) mRNA turnover rate limits siRNA and microRNA efficacy. Molecular Systems Biology, 6, 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber, N. , Nuhn, L. & Zentel, R. (2017) Cationic nanohydrogel particles for therapeutic oligonucleotide delivery. Macromolecular Bioscience, 17, e1700092. [DOI] [PubMed] [Google Scholar]
- Lee, Y.H. & Dean, R.A. (1994) Hydrophobicity of contact surface induces appressorium formation in Magnaporthe grisea . FEMS Microbiology Letters, 115, 71–75. [Google Scholar]
- Lykogianni, M. , Bempelou, E. , Karamaouna, F. & Aliferis, K.A. (2021) Do pesticides promote or hinder sustainability in agriculture? The challenge of sustainable use of pesticides in modern agriculture. Science of the Total Environment, 795, 148625. [DOI] [PubMed] [Google Scholar]
- Makinson, R.O. (2018) Myrtle rust reviewed: the impacts of the invasive pathogen Austropuccinia psidii on the Australian environment. Canberra: Plant Biosecurity Cooperative Research Centre. [Google Scholar]
- Makinson, R.O. & Conn, B.J. (2014) Puccinia psidii (Pucciniaceae – Eucalyptus rust, guava rust, myrtle rust) – a threat to biodiversity in the Indo‐Pacific region. Gardens' Bulletin Singapore, 66, 173–188. [Google Scholar]
- McDonald, B.A. & Linde, C. (2002) The population genetics of plant pathogens and breeding strategies for durable resistance. Euphytica, 124, 163–180. [Google Scholar]
- McDonald, M.C. , Renkin, M. , Spackman, M. , Orchard, B. , Croll, D. , Solomon, P.S. et al. (2019) Rapid parallel evolution of azole fungicide resistance in Australian populations of the wheat pathogen Zymoseptoria tritici . Applied and Environmental Microbiology, 85, e01908‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin, A.G. , Wytinck, N. , Walker, P.L. , Girard, I.J. , Rashid, K.Y. , De Kievit, T. et al. (2018) Identification and application of exogenous dsRNA confers plant protection against Sclerotinia sclerotiorum and Botrytis cinerea . Scientific Reports, 8, 7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTaggart, A.R. , Duong, T.A. , Le, V.Q. , Shuey, L.S. , Smidt, W. , Naidoo, S. et al. (2018). Chromium sequencing: the doors open for genomics of obligate plant pathogens. BioTechniques, 65, 253–257. [DOI] [PubMed] [Google Scholar]
- Miles, M.R. , Levy, C. , Morel, W. , Mueller, T. , Steinlage, T. , Van Rij, N. et al. (2007) International fungicide efficacy trials for the management of soybean rust. Plant Disease, 91, 1450–1458. [DOI] [PubMed] [Google Scholar]
- Miller, M.E. , Zhang, Y. , Omidvar, V. , Sperschneider, J. , Schwessinger, B. , Raley, C. et al. (2018) De novo assembly and phasing of dikaryotic genomes from two isolates of Puccinia coronata f. sp. avenae, the causal agent of oat crown rust. mBio, 9, e01650‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitter, N. , Worrall, E.A. , Robinson, K.E. , Li, P. , Jain, R.G. , Taochy, C. et al. (2017) Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nature Plants, 3, 16207. [DOI] [PubMed] [Google Scholar]
- Mosa, M.A. & Youssef, K. (2021) Topical delivery of host induced RNAi silencing by layered double hydroxide nanosheets: an efficient tool to decipher pathogenicity gene function of Fusarium crown and root rot in tomato. Physiological and Molecular Plant Pathology, 115, 101684. [Google Scholar]
- Nakayashiki, H. & Nguyen, Q.B. (2008) RNA interference: roles in fungal biology. Current Opinion in Microbiology, 11, 494–502. [DOI] [PubMed] [Google Scholar]
- Nerva, L. , Sandrini, M. , Gambino, G. & Chitarra, W. (2020) Double‐stranded RNAs (dsRNAs) as a sustainable tool against gray mold (Botrytis cinerea) in grapevine: effectiveness of different application methods in an open‐air environment. Biomolecules, 10, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, L.‐T. , Schmidt, H.A. , Von Haeseler, A. & Minh, B.Q. (2015) IQ‐TREE: a fast and effective stochastic algorithm for estimating maximum‐likelihood phylogenies. Molecular Biology and Evolution, 32, 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numata, K. , Ohtani, M. , Yoshizumi, T. , Demura, T. & Kodama, Y. (2014) Local gene silencing in plants via synthetic dsRNA and carrier peptide. Plant Biotechnology Journal, 12, 1027–1034. [DOI] [PubMed] [Google Scholar]
- Odell, D.E. & Smith, J. (1982) A simple method for spore germination studies. Bulletin of the British Mycological Society, 16, 159–161. [Google Scholar]
- Okonechnikov, K. , Golosova, O. & Fursov, M. (2012) Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics, 28, 1166–1167. [DOI] [PubMed] [Google Scholar]
- Panwar, V. , Jordan, M. , McCallum, B. & Bakkeren, G. (2018) Host‐induced silencing of essential genes in Puccinia triticina through transgenic expression of RNAi sequences reduces severity of leaf rust infection in wheat. Plant Biotechnology Journal, 16, 1013–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg, G.S. , Giblin, F.R. , McTaggart, A.R. , Guymer, G.P. , Taylor, H. , Ireland, K.B. et al. (2014) Puccinia psidii in Queensland, Australia: disease symptoms, distribution and impact. Plant Pathology, 63, 1005–1021. [Google Scholar]
- Pegg, G. , Taylor, T. , Entwistle, P. , Guymer, G. , Giblin, F. & Carnegie, A. (2017) Impact of Austropuccinia psidii (myrtle rust) on Myrtaceae‐rich wet sclerophyll forests in south East Queensland. PLoS One, 12, e0188058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendleton, A.L. , Smith, K.E. , Feau, N. , Martin, F.M. , Grigoriev, I.V. , Hamelin, R. et al. (2014) Duplications and losses in gene families of rust pathogens highlight putative effectors. Frontiers in Plant Science, 5, 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periyannan, S. (2018) Sustaining global agriculture through rapid detection and deployment of genetic resistance to deadly crop diseases. New Phytologist, 219, 45–51. [DOI] [PubMed] [Google Scholar]
- Pethybridge, S.J. & Nelson, S.C. (2015) Leaf Doctor: a new portable application for quantifying plant disease severity. Plant Disease, 99, 1310–1316. [DOI] [PubMed] [Google Scholar]
- Qi, T. , Zhu, X.G. , Tan, C.L. , Liu, P. , Guo, J. , Kang, Z.S. et al. (2018) Host‐induced gene silencing of an important pathogenicity factor PsCPK1 in Puccinia striiformis f. sp tritici enhances resistance of wheat to stripe rust. Plant Biotechnology Journal, 16, 797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao, L. , Lan, C. , Capriotti, L. , Ah‐Fong, A. , Nino Sanchez, J. , Hamby, R. et al. (2021) Spray‐induced gene silencing for disease control is dependent on the efficiency of pathogen RNA uptake. Plant Biotechnology Journal, 39, 1756–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . (2020) R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rochi, L. , Diéguez, M.J. , Burguener, G. , Darino, M.A. , Pergolesi, M.F. , Ingala, L.R. et al. (2018) Characterization and comparative analysis of the genome of Puccinia sorghi Schwein, the causal agent of maize common rust. Fungal Genetics and Biology, 112, 31–39. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Jiménez, L. , Polonio, Á. , Vielba‐Fernández, A. , Pérez‐García, A. & Fernández‐Ortuño, D. (2021) Gene mining for conserved, non‐annotated proteins of Podosphaera xanthii identifies novel target candidates for controlling powdery mildews by spray‐induced gene silencing. Journal of Fungi, 7, 735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter, B.D. & Innes, R.W. (2018) Extracellular vesicles as key mediators of plant–microbe interactions. Current Opinions in Plant Biology, 44, 16–22. [DOI] [PubMed] [Google Scholar]
- Saito, H. , Sakata, N. , Ishiga, T. & Ishiga, Y. (2022) Efficacy of RNA‐spray‐induced silencing of Phakopsora pachyrhizi chitin synthase genes to control soybean rust. Journal of General Plant Pathology, 88, 203–206. [Google Scholar]
- Saito, H. , Yamashita, Y. , Sakata, N. , Ishiga, T. , Shiraishi, N. , Usuki, G. et al. (2021). Covering soybean leaves with cellulose nanofiber changes leaf surface hydrophobicity and confers resistance against Phakopsora pachyrhizi . Frontiers in Plant Science, 12, 726565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoettle, A.W. & Sniezko, R.A. (2007) Proactive intervention to sustain high‐elevation pine ecosystems threatened by white pine blister rust. Journal of Forest Research, 12, 327–336. [Google Scholar]
- Šečić, E. & Kogel, K.‐H. (2021) Requirements for fungal uptake of dsRNA and gene silencing in RNAi‐based crop protection strategies. Current Opinion in Biotechnology, 70, 136–142. [DOI] [PubMed] [Google Scholar]
- Seifert, K.A. , Samson, R.A. , Dewaard, J.R. , Houbraken, J. , Levesque, C.A. , Moncalvo, J.M. et al. (2007) Prospects for fungus identification using CO1 DNA barcodes, with Penicillium as a test case. Proceedings of the National Academy of Sciences of the United States of America, 104, 3901–3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijen, T. , Fleenor, J. , Simmer, F. , Thijssen, K.L. , Parrish, S. , Timmons, L. et al. (2001) On the role of RNA amplification in dsRNA‐triggered gene silencing. Cell, 107, 465–476. [DOI] [PubMed] [Google Scholar]
- Soffe, R. , Altenhuber, N. , Bernach, M. , Remus‐Emsermann, M.N.P. & Nock, V. (2019) Comparison of replica leaf surface materials for phyllosphere microbiology. PLoS One, 14, e0218102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, X.S. , Gu, K.X. , Duan, X.X. , Xiao, X.M. , Hou, Y.P. , Duan, Y.B. et al. (2018) Secondary amplification of siRNA machinery limits the application of spray‐induced gene silencing. Molecular Plant Pathology, 19, 2543–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperschneider, J. , Jones, A.W. , Nasim, J. , Xu, B. , Jacques, S. , Zhong, C. et al. (2021) The stem rust fungus Puccinia graminis f. sp. tritici induces centromeric small RNAs during late infection that are associated with genome‐wide DNA methylation. BMC Biology, 19, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone, C.L. , McMahon, M.B. , Fortis, L.L. , Nuñez, A. , Smythers, G.W. , Luster, D.G. et al. (2012) Gene expression and proteomic analysis of the formation of Phakopsora pachyrhizi appressoria. BMC Genomics, 13, 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taning, C.N. , Arpaia, S. , Christiaens, O. , Dietz‐Pfeilstetter, A. , Jones, H. , Mezzetti, B. et al. (2020) RNA‐based biocontrol compounds: current status and perspectives to reach the market. Pest Management Science, 76, 841–845. [DOI] [PubMed] [Google Scholar]
- Tavares, S. , Ramos, A.P. , Pires, A.S. , Azinheira, H.G. , Caldeirinha, P. , Link, T. et al. (2014) Genome size analyses of Pucciniales reveal the largest fungal genomes. Frontiers in Plant Science, 5, 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres‐Martinez, S. & Ruiz‐Vázquez, R.M. (2017) The RNAi universe in fungi: a varied landscape of small RNAs and biological functions. Annual Review of Microbiology, 71, 371–391. [DOI] [PubMed] [Google Scholar]
- Varshney, R. , Thudi, M. , Aggarwal, R. & Börner, A. (2007) Genic molecular markers in plants: development and applications. In: Varshney, R.K. & Tuberosa, R. (Eds.) Genomics‐assisted crop improvement. Genomics approaches and platforms. New York: Springer, pp. 13–29. [Google Scholar]
- Weisenfeld, N.I. , Kumar, V. , Shah, P. , Church, D.M. & Jaffe, D.B. (2017) Direct determination of diploid genome sequences. Genome Research, 27, 757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham, H. , François, R. , Henry, L. , Müller, K. & RStudio . (2020) dplyr: a grammar of data manipulation. Available at: https://CRAN.R‐project.org/package=dplyr [Accessed 5th December 2022].
- Wynn, W.K. (1975) Appressorium formation over stomates by the bean rust fungus: response to a surface contact stimulus. Cytology and Histology, 66, 136–146. [Google Scholar]
- Wytinck, N. , Sullivan, D.S. , Biggar, K.T. , Crisostomo, L. , Pelka, P. , Belmonte, M.F. et al. (2020) Clathrin mediated endocytosis is involved in the uptake of exogenous double‐stranded RNA in the white mold phytopathogen Sclerotinia sclerotiorum . Scientific Reports, 10, 12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, C. , Downey, S. , Klages‐Mundt, N. , Ramachandran, S. , Chen, X. , Szabo, L. et al. (2015) Identification of promising host‐induced silencing targets among genes preferentially transcribed in haustoria of Puccinia . BMC Genomics, 16, 579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D.X. , Spiering, M.J. & Nuss, D.L. (2014) Characterizing the roles of Cryphonectria parasitica RNA‐dependent RNA polymerase‐like genes in antiviral defense, viral recombination and transposon transcript accumulation. PLoS One, 9, e108653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, L. , Zhu, J. , Liu, Z. , Wang, Z. , Zhou, C. & Wang, H. (2017) Host‐induced gene silencing of rice blast fungus Magnaporthe oryzae pathogenicity genes mediated by the brome mosaic virus. Genes, 8, 241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Coleosporium plumeriae Cy3‐CTP and Cy3‐dsRNA uptake assay. C. plumeriae urediniospores were incubated with 500 ng of Cy3‐labelled GFP dsRNA for 4 h and were then treated with 75 U of micrococcal nuclease. One biological replicate was set up for each treatment. The first image in each row is merged bright field (BF) and Cy3 fluorescence, the second is Cy3 lifetime, and the third is the corresponding phasor plot. Strong Cy3 fluorescence was seen in the germ tubes of spores treated with Cy3‐dsRNA, while only background fluorescence could be seen in the germ tubes of spores treated with Cy3‐CTP (negative control). Note that one nongerminated spore treated with Cy3‐CTP showed a strong fluorescence signal. Fluorescence lifetime imaging microscopy (FLIM, Datta et al., 2020) showed a difference in lifetime of Cy3 incorporated in dsRNA compared to in free CTP nucleotides. FLIM images were taken on a Leica Microsystems SP8 system, scale bars correspond to 20 μm
Figure S2 Selected Cy3 dsRNA_1 Z‐stack confocal images. The complete Z‐stack consisted of 33 images across a depth of 10.65 μm. Images were taken on a Leica Microsystems SP8 system, scale bars correspond to 20 μm
Figure S3 Examples of individual leaf photos used for disease coverage ratings in planta. Control (−dsRNA and GFP dsRNA) leaves both exhibit significant myrtle rust (MR) symptoms. One small MR lesion can be seen on the EF1‐a treatment leaf (yellow pustules to the right of the midvein). No MR symptoms can be seen on the β‐TUB treatment leaf
Table S1 Primers for in vitro synthesis of Austropuccinia psidii (Ap) and Coleosporium plumeriae (Cp)‐specific dsRNA. Transcripts per million (TPM) was calculated from unpublished RNA‐sequencing data
Data Availability Statement
Draft assemblies of all genomes sequenced in the present study are available at Zenodo (10.5281/zenodo.6650763) or at https://drive.google.com/drive/folders/1nDRWYLEO80R6CCxdnMzkaFY3DqUKn6Vo?usp=sharing. Raw sequencing data are available on request from ARM. All sequences used for analyses of genes in the RNAi pathway have been deposited in GenBank. Raw germination rate and disease assessment data that support the findings of this study are made available publicly through Dryad (10.5281/zenodo.7018192).


