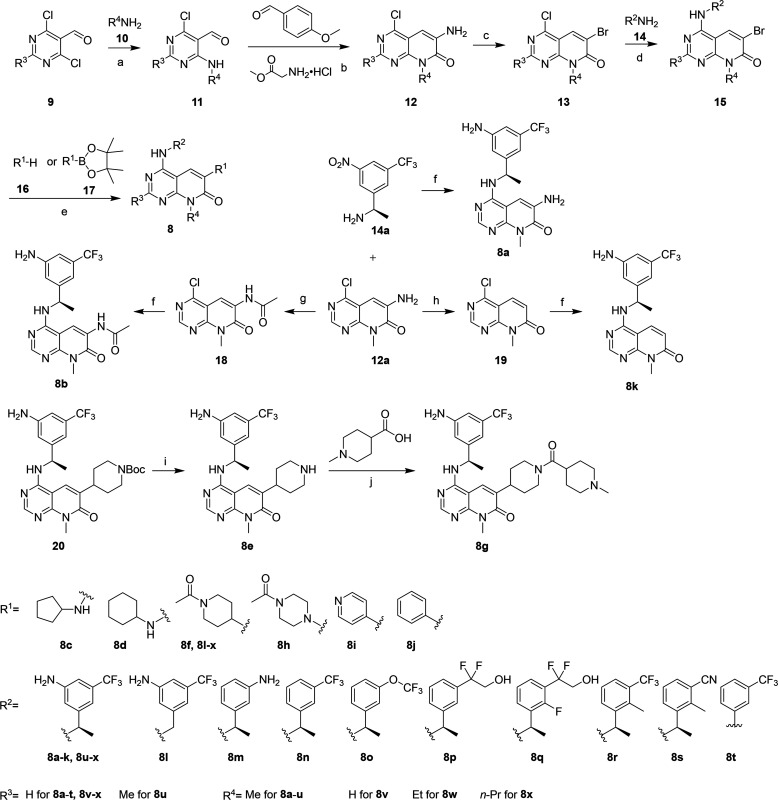
Scheme 1. Synthesis of Pyrido[2,3-d]pyrimidin-7-ones 8.
Reagents and conditions: (a) CHCl3, Et3N, 0 °C; rt, overnight. (b) Two steps: (1) Et3N, MeOH, rt, overnight; (2) 70% AcOH, 50 °C, 8 h. (c) CuBr, NaBr, t-BuONO, CH3CN, 0 °C; rt, overnight. (d) DIPEA, CsF, DMSO, 80 °C, overnight; for 8t, TFA, i-PrOH, 95 °C, overnight. (e) Two steps: (1) for 8c,d and 8h = amines 16, Pd(AcO)2, xantphos, Cs2CO3, 1,4-dioxane, 90 °C, overnight; for 8f, 8i,j, 8l–x, and 20 = reagents 17, Cs2CO3, Pd(dppf)Cl2, 1,4-dioxane, 80 °C, overnight; (2) for 8c,d, 8j, 8l–x, and 20 = Pd/C, H2, EtOAc, rt, overnight; for 8h,i = Fe, NH4Cl, EtOH, 90 °C, overnight. (f) Two steps: (1) 14a, DIPEA, CsF, DMSO, 80 °C, overnight; (2) Pd/C, H2, EtOAc, rt, overnight. (g) AcCl, DIPEA, CH3CN, reflux, overnight. (h) HCl (conc.), urea, NaNO2, H3PO2, THF, 0 °C, 5 h. (i) HCl (4 M in 1,4-dioxane), DCM, 0 °C; rt, overnight. (j) HATU, DIPEA, DMF, rt, overnight.