Abstract
During the early phase of the coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), diagnosis was difficult due to the diversity in symptoms and imaging findings and the variability of disease presentation. Pulmonary manifestations are reportedly the main clinical presentations of COVID-19 patients. Scientists are working hard on a myriad of clinical, epidemiological, and biological aspects to better understand SARS-CoV-2 infection, aiming to mitigate the ongoing disaster. Many reports have documented the involvement of various body systems and organs apart from the respiratory tract including the gastrointestinal, liver, immune system, renal, and neurological systems. Such involvement will result in diverse presentations related to effects on these systems. Other presentations such as coagulation defects and cutaneous manifestation may also occur. Patients with specific comorbidities including obesity, diabetes, and hypertension have increased morbidity and mortality risks with COVID-19.
Keywords: COVID-19, Non-pulmonary, Extrapulmonary, Clinical manifestations, Systemic disease
Core Tip: Pulmonary involvement was taking the upper hand during the early coronavirus disease 2019 pandemic, which was proven to be a rather multisystemic disease. Due to the helpful research efforts that could help in shifting the developing subject area and proper understanding of the nature of the disease for early diagnosis and controlling the spread of the infection.
INTRODUCTION
Since January 30, 2020, the World Health Organization has declared the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak as a public health emergency of international concern[1]. This pandemic, which emerged in China, has rapidly spread to affect almost the entire globe within a few weeks. Currently, researchers are working hard on a myriad of aspects, clinical, epidemiological, and biological, to better understand SARS-CoV-2 infection aiming at mitigating the ongoing disaster. As the number of confirmed coronavirus disease 2019 (COVID-19) patients is increasing daily by tens of thousands, clinicians are struggling to understand the possible damage which can complicate SARS-CoV-2 infection[2]. During the early phase of the COVID-19 pandemic, the diagnosis was difficult due to the diversity in symptoms and imaging findings and variability of disease presentation[3,4]. The Centers for Disease Control and Prevention has identified interim clinical presenting features for COVID-19 as fever (83%-99%), cough (59%-82%), fatigue (44%-70%), anorexia (40%-84%), shortness of breath (31%-40%), sputum production (28%-33%) and myalgias (11%-35%)[5]. According to a large Chinese cohort studying disease patterns in more than 44000 patients, disease severity ranged from mild constitutional symptoms and/or mild pneumonia in 81% to shortness of breath and hypoxemia, which complicates about 14% of patients. Acute respiratory distress syndrome (ARDS), respiratory failure, shock, and multi-organ failure occur in only 5% of the affected population[6]. Chest imaging is non-specific in many settings. Although COVID-19 patients can be identified through the detection of bilateral peripheral ground-glass opacities and air-space consolidation, mild or early diseases might lack radiological chest changes[7,8]. Consequently, The American College of Radiology denied computed tomography of the chest as the first-line test for SARS-COV-2. Medical practitioners realize that although the lungs are the most affected organs, the infection can extend to many other organs and systems, including the heart, blood vessels, kidneys, gut, and brain[2].
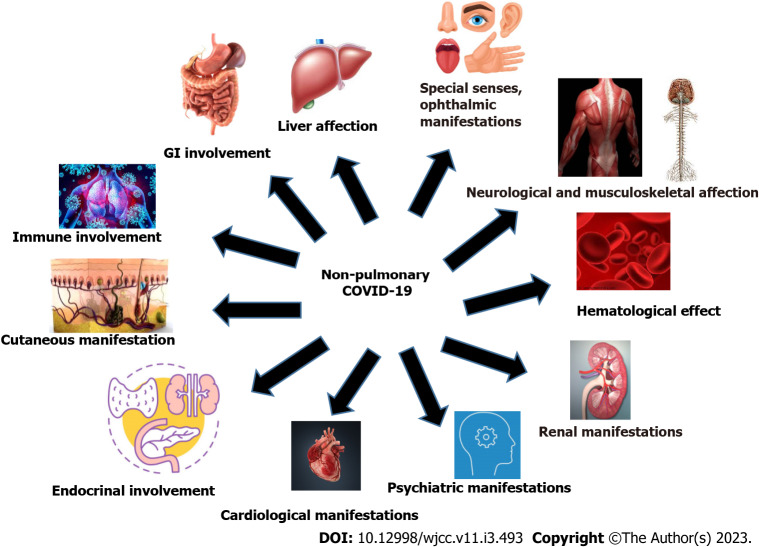
In this article, we focused on the non-pulmonary involvement in COVID-19 by reviewing the available evidence. Figure 1 summarizes the non-pulmonary involvement in COVID-19.
Figure 1.
Summarizes non-pulmonary involvement in coronavirus disease 2019. GI: Gastrointestinal; COVID-19: coronavirus disease 2019.
MATERIALS AND METHODS
The main objectives of the study were to stress the involvement of various body systems and organs in COVID-19 apart from the respiratory tract including the gastrointestinal (GI), liver, immune system, renal, and neurological systems.
Literature search
The literature search was conducted using the PubMed, Scopus, Web of Science (WOS), EBSCO, and Wiley databases. The following search items were used: ("non-pulmonary involvement OR "non-respiratory") AND ("COVID-19 OR "covid 19" OR "covid 19 associated") AND ("a systemic disease rather than a pure respiratory infection" OR "covid 19 associated multi-system inflammatory syndrome in adults" OR "covid 19 associated multi-system inflammatory" OR "covid 19 breakthrough infections" OR "covid 19 multi-system"), to retrieve relevant articles regardless the publishing year. For non-English articles, all relevant data were extracted from the English version of the abstract. The full text of these papers was translated into English to retrieve all other needed data. Reviews, meta-analyses, and all other articles containing non-original data were excluded from our review. All retrieved articles were screened and selected by two independent groups of authors (Mohamed El-Kassas and Mohamed Alboraie). Relevant data were extracted into a standardized data collection sheet by two independent authors (Mohamed Elbadry and Mohamed Eltabbakh).
Statistical analysis
Descriptive statistics were used for data analysis.
OBSERVATIONS AND DISCUSSION
GI involvement
In one of the first articles describing the clinical presentation of patients infected with SARS-CoV-2 from Wuhan, diarrhea was one of the clinical presentations[9]. In another study by Gu and his colleague from Shanghai, they described GI manifestations in the form of diarrhea, vomiting, and abdominal pain in COVID-19 patients[10]. With further studies, SARS-CoV-2 RNA was identified in the anal/rectal swabs and stool specimens of COVID-19 patients, even after virus clearance from the upper respiratory tract[11-13]. Genome sequences for SARS-CoV-2 showed that it has about 79.6% similarity to SARS-CoV, encoding and expressing the spike (S) glycoproteins, which help the virus enter human cells through binding to the angiotensin-converting enzyme 2 (ACE2) receptors[14]. ACE2 is highly expressed in type II alveolar cells (AT2) in the lungs and the GI tract, especially the small and large intestines[15]. Previous experience with the severe acute respiratory syndrome (SARS) epidemic in 2003 indicated that coronavirus has tropism in the GI tract. Electron microscopic examination of biopsies and autopsies revealed a rapid increase in the virus in the small intestine and the colon[16,17]. Likewise, Middle Eastern Respiratory Syndrome (MERS in 2013) caused by MERS-CoV can lead to intestinal infection since the human intestinal cells are strongly liable for and support this viral replication[18]. The high susceptibility of the GI tract (GIT) to coronavirus can explain the presence of diarrhea in a proportion of patients infected with COVID-19, despite being less frequent than in SARS. The viral nucleocapsid protein could be stained and identified inside the cytoplasm of epithelial cells of the stomach, duodenum, and rectum[19]. The initial autopsy belonged to an 85-year-old male with COVID-19 revealed areas of dilatation and narrowing of the small bowel[20]. One of the largest and most comprehensive studies evaluating GIT manifestations in COVID-19 patients from Wuhan, with 1141 cases admitted in a single hospital over 7 wk, showed that about 16% of patients presented with GI symptoms only. The most common symptoms were loss of appetite, nausea, and vomiting, which occurred in about 67% of the patients, diarrhea in 37%, and abdominal pain in 25%[21]. Another study from China stated that 48.5% of patients had GIT symptoms and the most common symptoms were as follows: anorexia (83.8%), diarrhea (29.3%), vomiting (8.1%), and abdominal pain (4.0%), with some patients having multiple symptoms. In this study, 7 patients had only GIT symptoms with no pulmonary symptoms, and those patients needed a longer hospital stay[22]. The same was reported by An et al[23], who described 9 adults with COVID-19 who initially presented with only GI manifestations. Five patients became febrile 2-4 d from the onset during their hospital admission, whereas the rest did not show any other symptoms. This should raise awareness about the possibility of SARS-CoV-2 infection in patients presenting with GIT manifestations during the pandemic, which may lead to the spread of infection if not detected early. The last point was specifically mentioned in a separate report describing a patient who suffered abdominal symptoms and was admitted to a surgical department, leading to the infection of more than 10 healthcare workers and 4 patients admitted in the same area[24]. In one of the largest review articles, Yuan and his colleagues analyzed data from 2023 patients, stating whether GI manifestations were present. The incidence of GIT manifestation was between 3% and 79% including anorexia (39.9%-50.2%), diarrhea (2%-49.5%), vomiting (3.6%-66.7%), nausea (1%-29.4%), abdominal pain (2.2%-6.0%), and GI bleeding (4%-13.7%). Both adults and children could present with GI manifestations without pulmonary symptoms. Fecal testing for viral ribonucleic acid (RNA) was as reliable as sputum in detecting SARS-CoV-2. In (36%-53%), fecal polymerase chain reaction (PCR) became positive 2-5 d following positive respiratory specimens. Fecal excretion continued after sputum excretion in (23%-82%) of patients for 1-11 d[25]. Another multicenter study that assessed patients with GIT manifestations in China reported that COVID-19 patients who suffered digestive symptoms had a longer time from symptom onset to admission and evidence of more laboratory derangements, including prolonged coagulation and higher liver enzymes, compared to those without GIT symptoms[22]. In another interesting case report, a 71-year-old woman developed abdominal pain and non-bloody diarrhea, followed by bloody diarrhea, nausea, vomiting, anorexia, and diffuse abdominal pain. Computed tomography showed severe colonic inflammation that was more in the ascending, transverse, and descending colon and had mild right pleural effusion. The patient underwent all investigations as infective diarrhea, which turned negative, and her sigmoidoscopy revealed mild mucosal inflammation. On the 4th day of hospital admission, the patient developed cough, and nasopharyngeal swabs turned positive for SARS-CoV-2[26]. The GI presentation of this case differed from all previously reported cases, thus highlighting the importance of taking all precautions when dealing with patients presenting with any GIT complaints during this pandemic.
Liver effects on patients with COVID-19
There are insufficient data for direct viral-related liver injury in COVID-19 cases. Data from one of the biggest centers in China showed that 2%-11% of COVID-19 cases had liver problems, and 14%-53% had abnormal levels of alanine aminotransferase and aspartate aminotransferase during the disease. Patients with severe COVID-19 had higher values of liver enzymes than mild cases with the disease[27]. Some patients from China have undergone autopsies or post-mortem tissue biopsies to identify the nature of liver injury. The first report was for a 50-year-old man who died from severe COVID-19, and the autopsy specimen showed moderate microvascular steatosis with a mild portal and lobular activity in the liver[28]. Other autopsies revealed hepatomegaly with dark and red hepatocyte degeneration and focal necrosis areas. Also, there was neutrophilic and lymphocytic infiltration in the portal area with congested hepatic sinuses and micro thrombosis. No pathological features of liver failure or injury of bile ducts could be detected in these cases[29].
The liver is considered a target for direct infection, as ACE2 is expressed abundantly in cholangiocytes, which leads to direct cytotoxicity[30]. COVID-19 may cause a severe inflammatory response, which leads to the immune-mediated damage of many organs, including the liver, which is evident by increased peripheral blood levels of many inflammatory markers such as ferritin and many cytokines such as interleukin 2 (IL-2) and IL-6. Also, sepsis is a common complication in severe COVID-19 cases, which may lead to hypoxic and ischemic liver injury, cholestasis, and hepatocellular injury due to severe inflammation. Drug-induced liver injury may be a common cause of liver involvement in COVID-19. Many national recommendations are using multiple antiviral drugs such as oseltamivir, umifenovir, chloroquine, tocilizumab, and lopinavir/ritonavir, which can induce liver injury. Most antipyretic drugs contain paracetamol, which is considered a known cause of hepatotoxicity in high doses[31]. Patients with pre-existing chronic liver disease may be more susceptible to liver damage during COVID-19 infection and its sequelae of immune-mediated damage with severe inflammation[32,33]. It seems that liver enzyme elevations are usually transient, and severe liver injury is rare with COVID-19. To the best of our knowledge, there are no documented deaths due to hepatic decompensation in cases without pre-existing liver disease. However, regular monitoring of the liver profile is warranted, especially in patients with severe COVID-19 infection[34].
Hematological effect of COVID-19
Hematological effects from SARS-CoV-2 are not a recent finding. Cells with ACE2 receptors might be infected first by the virus, including immune cells. Immune cells produce antibodies that can generate immune hemolysis when they contact red blood cells. Hemoglobin is then infected and attacked. The viral open reading frame 8 (ORF8) and surface glycoprotein can bind to porphyrin. Simultaneously, ORF1ab, ORF10, and ORF3a proteins can coordinate the attack of the beta chain of hemoglobin, leading to the dissociation of iron and the formation of porphyrin. This attack would cause a loss of hemoglobin, which is essential for carrying oxygen and carbon dioxide. The inability to frequently exchange carbon dioxide and oxygen has a too-intense poisoning and inflammatory effect on the lung cells, eventually resulting in characteristic ground glass-like lung images. The virus can also inhibit normal human heme anabolism[9,35].
That is why recent clinical studies believe that viral damage to the human body is systemic, not confined to the respiratory system. Additionally, overt disseminated intravascular coagulopathy (DIC) was found in 71.4% of non-survivors and 0.6% of survivors upon applying the validated International Society on Thrombosis and Hemostasis DIC score from a single center (the median time to DIC detection was 4 d). Based on these preliminary results, it was assumed that DIC can complicate COVID-19 pneumonia and be associated with mortality. Evidence of DIC, especially elevated D-dimer levels, may also be used to guide therapeutic interventions[36]. It was noticed that older patients with lymphopenia and high lactate dehydrogenase (LDH) levels on admission were associated with severe manifestations and required intensive care unit (ICU) admissions. Also, those patients who required ICU stay had a deeper nadir absolute lymphocytic count, nadir absolute monocyte count and nadir hemoglobin, and higher peak absolute neutrophil count and peak LDH levels compared to patients who did not require ICU[37]. Four possible mechanisms can explain why COVID-19 reduces blood lymphocyte levels: The virus might infect lymphocytes directly, resulting in death. The ACE2 coronavirus receptor is expressed on lymphocytes and may be directly targeted by the viruses[38]. The novel coronavirus virus might directly destroy lymphatic organs such as the thymus and spleen, and this hypothesis needs to be confirmed by pathological dissection in the future. Enhanced lymphocyte apoptosis due to inflammatory cytokines release (e.g., tumor necrosis factor alpha, IL-6, and other pro-inflammatory cytokines) could induce lymphocyte deficiency and lymphocyte inhibitions by metabolic molecules such as hyperlactic acidemia produced by metabolic disorders. In severe forms of COVID-19, patients had elevated blood levels of lactic acid, which might suppress lymphocyte proliferation[39]. The mechanisms mentioned above or beyond might work together or separately to cause lymphopenia, so further research is recommended. That is why lymphopenia is considered an effective and reliable indicator of disease severity and an indication for hospitalization in patients with COVID-19[40].
Renal manifestations of COVID 19
Renal involvement in COVID-19 (coronavirus-nephropathy) has a complex etiology. However, some studies suggest that acute kidney injury (AKI) does not complicate COVID-19, but when it occurs in COVID-19 patients, it is associated with higher morbidity and mortality and is a strong indicator of survival[41]. Renal affection is mutual in COVID-19 patients and manifests mainly with proteinuria in about 44% to 63% of patients, hematuria in 26.9% of patients, elevated serum creatinine (SCr) in 15.5% to 19%, and urea blood nitrogen (BUN) in 14.1% to 27% of the patients. AKI was also reported in 3.2% of infected individuals. Computed tomography (CT) scan of the kidneys illustrated that the renal parenchyma's inflammation and edema were evident in all patients. Renal failure in COVID-19 patients had a greater risk of in-hospital mortality. AKI, proteinuria, hematuria, raised plasma creatinine, and urea nitrogen were evident predictors of in-hospital patients’ mortality as well[42,43]. Many mechanisms have been suggested to explain how SARS-CoV-2 impacts renal functions, one of which is attributed to dehydration, which may result from fever; and decreased fluid intake, which leads to a reduction of glomerular filtration rate and causes AKI. This can be reversible in early stages by fluid therapy. However, if dehydration leads to hypoperfusion and ischemia, as in cases of cytokine storm, sepsis and shock, acute tubular necrosis might supervene[44,45]. Rhabdomyolysis and hypoxia are other possibilities, as well as direct virus invasion to the renal tubular cells, interstitium, or glomeruli with direct cytopathic effect. Coronaviruses have a three-dimensional spike protein structure, which is closely bound to human cell receptor 2, where it enters into the cells via ACE2 receptors, which are vastly expressed in the renal cells. This explains how the renal cells are targeted and infected by SARS-CoV-2. Although the virus-induced glomerulopathy in the coronaviruses family was low, immune complexes deposition of viral particles or virus-induced specific immunological abnormalities is still possible[46,47]. However, another opinion was that COVID-19 does not induce AKI or deterioration of the renal condition in patients with chronic kidney disease (CKD). In a study including 116 cases confirmed with COVID-19, only 10.8% of patients without CKD showed a mild increase in BUN or SCr, and these elevations were all less than 26 μmol/L within 48 h, which does not match with the AKI standard definition. Only 7.2% of the patients without CKD showed mild albuminuria and gradually returned to normal without needing specific treatment[41].
Cardiological manifestations of COVID-19
The cardiovascular system is sometimes involved during the COVID-19 course by different proposed mechanisms, such as direct myocardial injury by binding to ACE2 receptors, which leads to alteration of signaling pathways that result in acute myocardial injury. Another supposed mechanism is the systemic inflammatory response and cytokine storm, which ultimately leads to multiple end-organ damage proven by the high circulatory levels of proinflammatory cytokines in patients with critical COVID-19. This may also lead to increased coronary blood flow with increased shear stress that can precipitate plaque rupture and increase vascular thrombosis leading to acute myocardial infarction[48,49]. The hypoxia resulting from acute lung injury may cause a significant imbalance of the oxygen demand-supply ratio to the cardiac muscle and increases cardio-metabolic demand. Electrolyte disturbance, especially hypokalemia (of particular concern in patients with COVID-19), adversely affects various therapies, such as antiviral drugs, which affect QT interval prolongation and corticosteroids, and other therapies can also have deleterious effects on the cardiovascular system[50]. Although cardiovascular risk factors do not increase the chance of getting SARS-CoV-2 infection, they inversely affect the prognosis of COVID-19. These risk factors include diabetes, cardio-cerebrovascular disease, and hypertension, and they were associated with a 2-3 fold greater risk of severe disease, respectively, or requiring ICU admission[6]. Acute myocardial injury is the most commonly described cardiac presentation in COVID-19. Different biomarkers cut-offs and/or electrocardiographic abnormalities have been used to define acute cardiac injury. The most commonly used definition is an elevation of high-sensitivity cardiac troponin above the 99th percentile upper reference limit. The incidence of acute myocardial injury is roughly estimated to be 8%-12% among positive cases of SARS-CoV-2 with a robust unfavorable prognosis of COVID-19. The incidence of ST-segment elevation myocardial infarction in COVID-19 patients seems to be lower, as well as the incidence of left ventricular systolic dysfunction, acute left ventricular failure, and cardiogenic shock. Heart failure has been reported in 52% of COVID-19 patients who subsequently died and 12% of patients discharged from the hospital. Tachy- and bradyarrhythmias were also reported in the severe and morbid form of the disease requiring ICU admission, while acute coronary events and left ventricular systolic dysfunction were not reported and seemed to have very low incidence[51-53]. Interestingly, although myocardial injury is significantly associated with the fatal outcome of COVID-19, the prognosis of patients with underlying cardiovascular disease (excluding myocardial) injury is relatively favorable[54].
Endocrinal involvement in COVID-19
Diabetes, hypertension, and metabolic syndrome significantly impact morbidity and mortality in COVID-19. Hyperglycemia and diabetes are associated with suppression of the immune state and increase the risk of infectious diseases. About 51% of COVID-19 patients reportedly have hyperglycemia, similar to what has been observed with other viral infections such as SARS and MERS. The transient pancreatic cell function impairment can explain the mechanism of hyperglycemia in this situation by the virus itself or by modulation in glucose metabolism. The impact of hyperglycemia in SARS-CoV-2 infection cannot be underestimated due to its effect on the immune status and its further complications[55]. About 50%-60% of impaired glucose tolerance and diabetic patients have a higher incidence of pulmonary infections. Diabetic patients carry a high risk of getting SARS-CoV-2 infection. A study from Wuhan concluded that other diseases accompanied 32% of the infected cases; the highest was for diabetes in 20% of cases, followed by hypertension in 15% and cardiovascular disease in 15% of cases[9]. Another study retrospectively analyzed data from 138 COVID-19 patients and found that 46.4% had one or more underlying diseases, of which diabetes was represented in 10% of cases and 22.2% of ICU cases[24]. Unsurprising that patients with diabetes have a high rate of mortality among SARS-CoV-2 infected patients, it reached 77.7% of critically ill COVID-19 patients[56]. Also, of the 72,314 reported cases of COVID-19 in the Chinese Center for Disease Control and Prevention, diabetic patients had a high mortality rate (7.3% in diabetes mellitus vs 2.3% overall)[6].
Generally, there are a lack of data about the effect of obesity on COVID-19 patients. However, some hospitals in Spain showed that obese patients infected with SARS-CoV-2 were more susceptible to severe ARDS, respiratory failure, and even death; this can be explained by sleep apnea syndrome and surfactant dysfunction that occur with severe obesity[57]. Undernourished patients are more susceptible to infection with SARS-CoV-2. Also, COVID-19 itself can lead to malnutrition, which affects outcome and prognosis; therefore, COVID-19 outcome could be improved with improved nutritional status[58].
Unfortunately, there are no currently available data about the outcome of COVID-19 patients with adrenal insufficiency; however, it is expected they may have a high risk for acquiring SARS-CoV-2 infection and high incidence of mortality due to defects in their natural immunity, neutrophils, and natural killer cell action[59]. Similarly, few published data are available about the effect of SARS-CoV infection on male gonads and the risk of infertility. Germ cell destruction, few or no spermatozoon in the seminiferous tubules, a thickened basement membrane, and leukocyte infiltration were found in some SARS-COV-infected patients. This may be explained by sharing the same ACE2 receptor to enter the host cells for reproduction and transmission[60]. Whether a similar effect can occur in SARS-CoV-2 infected patients; needs more research to explore.
Immune involvement in COVID-19
Both innate and adaptive immunity are involved in SARA-CoV-2 infection, with lymphopenia being dominant; however, the severely uncontrollable inflammatory response with markedly elevated levels of proinflammatory cytokines such as IL-6 and IL-1β, as well as IL-2, IL-8, and IL-17, known as cytokine storm, has been seen in severely infected COVID-19 patients. This cytokine storm leads to multiple unwanted harmful effects that can be manifested with severe tissue damage, extensive pulmonary destruction, respiratory failure, multi-organ failure, and even death. In addition to elevated levels of proinflammatory cytokines, complement activation has also been seen to add another immune-based pathology associated with COVID-19; this may contribute to further hope for using complement inhibitors to decrease inflammatory damage[61].
SARS-CoV-2-specific immunoglobulin G (IgG) and IgM antibodies have been detected in high titer upon analysis of 222 and 173 COVID-19-infected patients; this may be explained by an antibody-mediated immune mechanism that has also been observed with many other viral infections. Hopefully, this can be helpful for antibody-based therapies and vaccine development[62].
Cutaneous manifestation in COVID-19 patients
Available data about the skin manifestations of COVID-19 are scarce. COVID-19 patients may initially present with a skin rash, which may be misdiagnosed as other common skin diseases[9]. A study conducted in Italy collected data from 88 COVID-19 patients; 20.4% developed cutaneous manifestations. Eight patients developed skin manifestations at the onset and ten patients after hospitalization. The developed cutaneous manifestations were variable and included: erythematous rash, widespread urticaria, and chickenpox-like vesicles. Lesions were more evident on the trunk. Most of the lesions were itchy and usually healed in a few days. No correlation was observed between disease severity and cutaneous symptoms. This study reported that skin manifestations recorded in COVID-19 patients are similar to those in common viral infections[63]. A case report from Thailand presented a patient with a petechial skin rash that was first diagnosed as dengue fever because of the endemicity of Dengue in Thailand and the presence of thrombocytopenia, later on, the patient developed respiratory symptoms and all viral causes that might be associated with fever, rash, and the respiratory problem was excluded by laboratory investigation. The final diagnosis of the SARS-COV-2 infection was made using real-time PCR[64]. Urticaria also was reported as a common skin manifestation associated with COVID-19 in Chinese patients[65,66].
Neurological and musculoskeletal systems are affected in COVID-19
Due to the similarities in structure and modes of transmission with SARS-CoV and MERS-CoV, SARS-CoV-2 showed growing evidence of affinity affecting the central nervous system and causing other neurological manifestations. Many early reported data described symptoms of neurological affection, commonly headache, which was severe and intolerable in many cases, dizziness, and unsteadiness of gait[67]. In the first retrospective observational study about the neurological involvement among 214 hospitalized patients in Wuhan, headache was the most common neurologic symptom, affecting 25% of the patients, followed by dizziness in 17%, then a disturbance in their level of consciousness in 8% of cases and acute cerebrovascular events in 3%. Less commonly, ataxia and convulsions occurred in 0.5% of each[68]. In another single-center retrospective observational study, including 221 patients, acute ischemic strokes were found in 5% of patients, with cerebral venous sinus thrombosis in 0.5% and cerebral hemorrhage in 0.5% of cases without the mention of other neurologic deficits. It is worth considering that almost all of these patients were old with accompanying risk factors like hypertension and diabetes mellitus[69]. It was suggested that metabolic and electrolyte imbalances, along with the accompanying hypoxia and/or multiple organ failure in critically ill patients, might represent a plausible explanation for altering mental status[70]. The presence of seizures ranging from subtle subclinical forms to clinically overt seizures and status epilepticus in many patients points to the possibility of having non-convulsive status epilepticus as an explanation, and hence the importance of electroencephalogram monitoring of such patients and the application of Salzburg Consensus Criteria for Non-Convulsive Status Epilecticus[71].
Moreover, some reports have linked Guillain-Barré syndrome and post-infectious acute myelitis to COVID-19. A similar para-infectious association with the Zika virus encouraged the suggestion of a phenomenon with COVID-19 instead of the classic post-infectious form[72,73]. The current piling data and reports of COVID-19 render the pandemic's central nervous system and other neurological manifestations more than just an epiphenomenon as concrete evidence is accumulated. The first reports of substantial evidence include a case of a 24-years-old man who presented with fever and fatigue. COVID-19 RNA was not detected in his nasopharyngeal swab until the 9th day when he was found unconscious and soaking in his vomit with evident neck stiffness and was diagnosed with meningeo-encephalitis. Despite the negative nasopharyngeal swab, COVID-19 RNA was found in his CSF fluid, while his serum varicella-zoster IgM and anti-herpes simplex virus type 1 were negative. Furthermore, magnetic resonance imaging showed hyperintensity signals in the right lateral ventricle, hippocampus, and right mesial temporal lobe, linking SARS-CoV-2 with his illness[74].
Psychiatric manifestations
During the COVID-19 pandemic, additional anxiety results from fear of acquiring infection, especially in the presence of uncertainty, whether other modes of transmission than droplet infection exist. The uncertain incubation period of the virus and its possible asymptomatic transmission can also cause additional stress. The obligation to social distancing in many countries worldwide resulted in changes in national behavioral patterns and the shutdown of the usual day-to-day functioning[75]. Additionally, reports of shortages in medical protective equipment, medical staff, and hospital beds have raised major worries worldwide. Lastly, a unique “infodemic” due to a plethora of information from unofficial and unreliable sources poses a significant risk to public mental health during this crisis[76].
The spectrum of psychological distresses can be classified into immediate and long-term effects. Immediate stress during an infectious disease outbreak can include fear and worriedness about a person’s health and the health of his family; changes in sleep or eating patterns; difficulty sleeping or concentrating; worsening of chronic health problems; worsening of mental health conditions; and increased use of alcohol, tobacco, or illicit drugs[77]. Panic disorders (resulting from fear of the unknown and the unpredicted) and infomania (the obsessive demand to frequently check media) can also be among the immediate effects. Stigma, xenophobia, and racism related to COVID-19 may also occur. Increased fear and suspicion towards people from China and other countries in which SARS-CoV-2 had widely spread led to social stigmatization and refusal to house, employ, or provide health care for certain races. Theories of conspiracy (mistrust, the detection of real and hidden designs, and the meaning behind the apparent causes) are also present. They have widely spread during the COVID-19 pandemic as different accusations of the virus as a biological war or designed to create an economic crisis are widespread[78]. Studies assessing mental health during the SARS outbreak in 2003 found that the main psychiatric problems were adjustment reactions with increased anxiety levels. Studies have also shown that 10%-35% of SARS survivors reported having features of anxiety, depression, or both during the early recovery phase[79].
Long-term psychiatric consequences of SARS-CoV-2 are still unknown; guided by the last SARS epidemic in 2003, a significant impact on mental health can be expected. In 2003, posttraumatic stress disorder was the most typical psychiatric condition as its cumulative incidence rate of 30 mo after SARS was (47.8%) and then depressive disorders were the second most common impact[80]. Healthcare workers (HCWs) carrying the responsibility to provide care to infected patients and to search for the best therapies for unknown dangerous pathogens are put under significant stress in addition to being exposed to infection. Exposure to infected patients necessitates the social isolation of HCWs with its added psychiatric consequences. When HCWs become infected and turn out to be patients, this change in their role leads to behavior adjustment challenges, frustration, and feeling of helplessness[81].
Special senses
Anosmia and ageusia (loss of smell and taste, respectively) were reported as frequent symptoms in patients with COVID-19[82]. In Italy, of 59 patients with COVID-19, 34% self-reported either smell or taste alteration, and 19% reported both[83]. The same finding was reported by a European multicenter cross-sectional study that showed that out of 417 mild-to-moderate COVID-19 patients, 85.6% reported smell dysfunction, and 88% reported taste dysfunction[82]. An American study on 237 COVID-19 patients showed that anosmia was experienced in 73% of subjects before COVID-19 diagnosis, and it was the initial symptom in about 27% of them[84]. Quantitative smell testing of confirmed 60 COVID-19 patients showed that 98% have some smell dysfunction but not always anosmia[85]. Considering these symptoms as characteristic early diagnostic features of COVID-19 is controversial as the earlier data from china did not stress olfactory or taste disorders in COVID-19 patients. The increasing evidence on the importance of olfactory and gustatory symptoms as a precedent event to the full-blown clinical disease suggests its use as a clinical screening tool to determine the need for early testing, treatment, or quarantine of asymptomatic individuals.
Ophthalmic manifestations
COVID-19 transmission through the ocular route remains uncertain[86]. Animal studies showed that coronaviruses, including SARS-CoV-2, can produce a broad spectrum of ocular manifestations from diseases that involve the anterior segment, like conjunctivitis and anterior uveitis, to posterior segment conditions like retinitis and optic neuritis[87]. The human eye has its intraocular renin-angiotensin system, as ACE2 was detected in the aqueous humor[88]. However, the expression of ACE2 in the conjunctiva or cornea is not well documented. So, ocular infection through ACE2 should be further investigated. A Chinese case series showed that about one-third of patients with COVID-19 had ocular abnormalities. Their ocular manifestations varied between epiphora, conjunctival congestion, or chemosis and were encountered in patients with advanced systemic manifestations[89]. Few case reports have described acute conjunctivitis as a presenting symptom of COVID-19 patients[90,91].
Audio vestibular manifestations
Viral infections are well known for causing sensorineural hearing loss. The evidence of inner ear involvement in COVID-19 patients is scarce and needs further studies. In a recent pilot study that explored the audiological profile of asymptomatic COVID-19 PCR-positive cases, the authors reported a significant worsening of the high-frequency pure-tone thresholds, as well as the transient, evoked otoacoustic emissions amplitudes. This indicates suspected deleterious effects of COVID-19 on the hair cells in the cochlea, but larger studies and follow-ups of these patients after recovery are pivotal to determining the exact mechanism and fate of these effects[92]. Furthermore, Fidan and his colleagues reported a 35-year-old female patient with otalgia and tinnitus. None of the classic COVID-19 symptoms with no comorbidities upon investigation proved to have acute otitis media, bilateral lung involvement in chest X-ray, and positive RT-PCR result for SARS-CoV-2[93].
CONCLUSION
In this review article, we highlighted multisystemic involvement of COVID-19 infection. Involvement of various body systems and organs apart from the respiratory tract, including the GI, liver, immune system, renal, and neurological systems have been reported with COVID-19 infection.
ACKNOWLEDGEMENTS
The authors acknowledge the tremendous effort and sacrifice of the medical staff working in COVID-19 quarantine hospitals worldwide.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: July 14, 2022
First decision: October 24, 2022
Article in press: January 5, 2023
Specialty type: Medicine, research and experimental
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Navarro-Alvarez N, Mexico; Patel MV, India S-Editor: Liu GL L-Editor: Filipodia P-Editor: Liu GL
Contributor Information
Mohamed El-Kassas, Department of Endemic Medicine, Faculty of Medicine, Helwan University, Cairo 11731, Egypt.
Mohamed Alboraie, Department of Internal Medicine, Al-Azhar University, Cairo 11884, Egypt.
Mohamed Elbadry, Department of Endemic Medicine, Faculty of Medicine, Helwan University, Cairo 11731, Egypt.
Reem El Sheemy, Department of Tropical Medicine, Minia Faculty of Medicine, Minia University, Minia 61511, Egypt.
Mohamed Abdellah, Department of Internal Medicine, Al-Azhar University, Cairo 11884, Egypt.
Shimaa Afify, Department of Gastroenterology, National Hepatology and Tropical Medicine Research Institute, Cairo 11451, Egypt.
Ahmad Madkour, Department of Endemic Medicine, Faculty of Medicine, Helwan University, Cairo 11731, Egypt.
Mariam Zaghloul, Department of Hepatology, Gastroenterology and Infectious Diseases, Faculty of Medicine, Kafrelsheikh University, Kafrelsheikh 33511, Egypt.
Abeer Awad, Department of Internal Medicine, Hepatogastroenterology Unit, Kasr Al-Ainy School of Medicine, Cairo 11451, Egypt. abeer.abdellatif86@gmail.com.
Mohamed-Naguib Wifi, Department of Internal Medicine, Hepatogastroenterology Unit, Kasr Al-Ainy School of Medicine, Cairo 11451, Egypt.
Amira Al Balakosy, Department of Tropical Medicine, Ain Shams University, Cairo 11451, Egypt.
Mohamed Eltabbakh, Department of Tropical Medicine, Ain Shams University, Cairo 11451, Egypt.
References
- 1.World Health Organization. (2020) Novel coronavirus (2019-nCoV) situation reports. [Internet] [accessed 16 May 2020]. Available from: https://www.who.int/emergencies/diseases/
- 2.Wadman M, Couzin-Frankel J, Kaiser J, Matacic C. How does coronavirus kill? Science AAAS. 2020 doi: 10.1126/science.368.6489.356. [DOI] [PubMed] [Google Scholar]
- 3.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Center for Disease Control and Prevention. Management of Patients with Confirmed 2019-nCoV. [Internet] [accessed May 16 2020]. 4/21/2020. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html .
- 6.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 7.Bai HX, Hsieh B, Xiong Z, Halsey K, Choi JW, Tran TML, Pan I, Shi LB, Wang DC, Mei J, Jiang XL, Zeng QH, Egglin TK, Hu PF, Agarwal S, Xie FF, Li S, Healey T, Atalay MK, Liao WH. Performance of Radiologists in Differentiating COVID-19 from Non-COVID-19 Viral Pneumonia at Chest CT. Radiology. 2020;296:E46–E54. doi: 10.1148/radiol.2020200823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu J, Han B, Wang J. COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission. Gastroenterology. 2020;158:1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang W, Du RH, Li B, Zheng XS, Yang XL, Hu B, Wang YY, Xiao GF, Yan B, Shi ZL, Zhou P. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK Washington State 2019-nCoV Case Investigation Team. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Y, Li X, Zhu B, Liang H, Fang C, Gong Y, Guo Q, Sun X, Zhao D, Shen J, Zhang H, Liu H, Xia H, Tang J, Zhang K, Gong S. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532:107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 16.Hung IF, Cheng VC, Wu AK, Tang BS, Chan KH, Chu CM, Wong MM, Hui WT, Poon LL, Tse DM, Chan KS, Woo PC, Lau SK, Peiris JS, Yuen KY. Viral loads in clinical specimens and SARS manifestations. Emerg Infect Dis. 2004;10:1550–1557. doi: 10.3201/eid1009.040058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leung WK, To KF, Chan PK, Chan HL, Wu AK, Lee N, Yuen KY, Sung JJ. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011–1017. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou J, Li C, Zhao G, Chu H, Wang D, Yan HH, Poon VK, Wen L, Wong BH, Zhao X, Chiu MC, Yang D, Wang Y, Au-Yeung RKH, Chan IH, Sun S, Chan JF, To KK, Memish ZA, Corman VM, Drosten C, Hung IF, Zhou Y, Leung SY, Yuen KY. Human intestinal tract serves as an alternative infection route for Middle East respiratory syndrome coronavirus. Sci Adv. 2017;3:eaao4966. doi: 10.1126/sciadv.aao4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao D, Yao F, Wang L, Zheng L, Gao Y, Ye J, Guo F, Zhao H, Gao R. A Comparative Study on the Clinical Features of Coronavirus 2019 (COVID-19) Pneumonia With Other Pneumonias. Clin Infect Dis. 2020;71:756–761. doi: 10.1093/cid/ciaa247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Q, Wang RS, Qu GQ, Wang YY, Liu P, Zhu YZ, Fei G, Ren L, Zhou YW, Liu L. Gross examination report of a COVID-19 death autopsy. Fa Yi Xue Za Zhi. 2020;36:21–23. doi: 10.12116/j.issn.1004-5619.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Erratum for the Research Article: "Circulating tumor DNA methylation profiles enable early diagnosis, prognosis prediction, and screening for colorectal cancer" by H. Luo, Q. Zhao, W. Wei, L. Zheng, S. Yi, G. Li, W. Wang, H. Sheng, H. Pu, H. Mo, Z. Zuo, Z. Liu, C. Li, C. Xie, Z. Zeng, W. Li, X. Hao, Y. Liu, S. Cao, W. Liu, S. Gibson, K. Zhang, G. Xu, R.-h. Xu. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.aax7533. [DOI] [PubMed] [Google Scholar]
- 22.Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu L. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.An P, Ji M, Ren H, Su J, Ding NS, Kang J, Yin A, Zhou Q, Shen L, Zhao L, Jiang X, Xiao Y, Tan W, Lv X, Li J, Liu S, Zhou J, Chen H, Xu Y, Liu J, Chen M, Cao J, Zhou Z, Tan S, Yu H, Dong W, Ding Y. Prevention of COVID-19 in patients with inflammatory bowel disease in Wuhan, China. Lancet Gastroenterol Hepatol. 2020;5:525–527. doi: 10.1016/S2468-1253(20)30121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51:843–851. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carvalho A, Alqusairi R, Adams A, Paul M, Kothari N, Peters S, DeBenedet AT. SARS-CoV-2 Gastrointestinal Infection Causing Hemorrhagic Colitis: Implications for Detection and Transmission of COVID-19 Disease. Am J Gastroenterol. 2020;115:942–946. doi: 10.14309/ajg.0000000000000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chai X, Hu L, Zhang Y, et al. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. bioRxiv; 2020. [Google Scholar]
- 31.Caines A, Moonka D. Drug Hepatotoxicity: Causality Assessment. Clin Liver Dis. 2020;24:25–35. doi: 10.1016/j.cld.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60:2099–2108. doi: 10.1002/hep.27406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Fan JG. Characteristics and Mechanism of Liver Injury in 2019 Coronavirus Disease. J Clin Transl Hepatol. 2020;8:13–17. doi: 10.14218/JCTH.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun J, Aghemo A, Forner A, Valenti L. COVID-19 and liver disease. Liver Int. 2020;40:1278–1281. doi: 10.1111/liv.14470. [DOI] [PubMed] [Google Scholar]
- 35.Lansiaux E, Pébaÿ PP, Picard JL, Son-Forget J. COVID-19: beta-thalassemia subjects immunised? Med Hypotheses. 2020;142:109827. doi: 10.1016/j.mehy.2020.109827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arachchillage DRJ, Laffan M. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1233–1234. doi: 10.1111/jth.14820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan BE, Chong VCL, Chan SSW, Lim GH, Lim KGE, Tan GB, Mucheli SS, Kuperan P, Ong KH. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020;95:E131–E134. doi: 10.1002/ajh.25774. [DOI] [PubMed] [Google Scholar]
- 38.Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T, Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G, Hoves S, Renner K, Timischl B, Mackensen A, Kunz-Schughart L, Andreesen R, Krause SW, Kreutz M. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 40.Tan L, Wang Q, Zhang D, Ding J, Huang Q, Tang YQ, Miao H. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, Li X, Chen H, Yan S, Li D, Li Y, Gong Z. Coronavirus Disease 19 Infection Does Not Result in Acute Kidney Injury: An Analysis of 116 Hospitalized Patients from Wuhan, China. Am J Nephrol. 2020;51:343–348. doi: 10.1159/000507471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balawender K, Pliszka A, Krowiak A, Sito M, Grabarek BO, Boroń D. Does SARS-CoV-2 Affect Male Urogenital System? Curr Pharm Biotechnol. 2022;23:1792–1799. doi: 10.2174/1389201023666220307102147. [DOI] [PubMed] [Google Scholar]
- 43.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Menachery VD, Yount BL Jr, Debbink K, Agnihothram S, Gralinski LE, Plante JA, Graham RL, Scobey T, Ge XY, Donaldson EF, Randell SH, Lanzavecchia A, Marasco WA, Shi ZL, Baric RS. Corrigendum: A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat Med. 2016;22:446. doi: 10.1038/nm0416-446d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valizadeh R, Baradaran A, Mirzazadeh A, Bhaskar LVKS. Coronavirus-nephropathy; renal involvement in COVID-19. J Renal Inj Prev. 2020;9:e18. [Google Scholar]
- 48.Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, Bi Z, Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiong TY, Redwood S, Prendergast B, Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J. 2020;41:1798–1800. doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao LL, Gaffney LK, Marcus C. Hypokalemia-Induced Rhabdomyolysis in a Child with Autism Affected by the COVID-19 Pandemic. J Dev Behav Pediatr. 2022;43:e356–e360. doi: 10.1097/DBP.0000000000001035. [DOI] [PubMed] [Google Scholar]
- 51.Bonow RO, Fonarow GC, O'Gara PT, Yancy CW. Association of Coronavirus Disease 2019 (COVID-19) With Myocardial Injury and Mortality. JAMA Cardiol. 2020;5:751–753. doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- 52.Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med. 2020;58:1131–1134. doi: 10.1515/cclm-2020-0198. [DOI] [PubMed] [Google Scholar]
- 53.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr. 2020;14:247–250. doi: 10.1016/j.dsx.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ilias I, Zabuliene L. Hyperglycemia and the novel Covid-19 infection: Possible pathophysiologic mechanisms. Med Hypotheses. 2020;139:109699. doi: 10.1016/j.mehy.2020.109699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puig-Domingo M, Marazuela M, Giustina A. COVID-19 and endocrine diseases. A statement from the European Society of Endocrinology. Endocrine. 2020;68:2–5. doi: 10.1007/s12020-020-02294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burgos R, García-Almeida JM, Matía-Martín P, Palma S, Sanz-Paris A, Zugasti A, Alfaro JJ, Fullana AA, Continente AC, Chicetru MJ, Malpartida KG, Faes ÁG, Sánchez VG, López ML, Ortega AJM, Roldán JO, Moreno CS, Llanos PS. Malnutrition management of hospitalized patients with diabetes/hyperglycemia and COVID-19 infection. Rev Endocr Metab Disord. 2022;23:205–213. doi: 10.1007/s11154-022-09714-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bancos I, Hazeldine J, Chortis V, Hampson P, Taylor AE, Lord JM, Arlt W. Primary adrenal insufficiency is associated with impaired natural killer cell function: a potential link to increased mortality. Eur J Endocrinol. 2017;176:471–480. doi: 10.1530/EJE-16-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu J, Qi L, Chi X, Yang J, Wei X, Gong E, Peh S, Gu J. Orchitis: a complication of severe acute respiratory syndrome (SARS) Biol Reprod. 2006;74:410–416. doi: 10.1095/biolreprod.105.044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, Wang X, Yuan J, Li T, Li J, Qian S, Hong C, Wang F, Liu Y, Wang Z, He Q, Li Z, He B, Zhang T, Fu Y, Ge S, Liu L, Zhang J, Xia N, Zhang Z. Antibody Responses to SARS-CoV-2 in Patients With Novel Coronavirus Disease 2019. Clin Infect Dis. 2020;71:2027–2034. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212–e213. doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- 64.Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol. 2020;82:e177. doi: 10.1016/j.jaad.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu S, Lin J, Zhang Z, Xiao L, Jiang Z, Chen J, Hu C, Luo S. Alert for non-respiratory symptoms of coronavirus disease 2019 patients in epidemic period: A case report of familial cluster with three asymptomatic COVID-19 patients. J Med Virol. 2021;93:518–521. doi: 10.1002/jmv.25776. [DOI] [PubMed] [Google Scholar]
- 66.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao YD. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 67.Wang HY, Li XL, Yan ZR, Sun XP, Han J, Zhang BW. Potential neurological symptoms of COVID-19. Ther Adv Neurol Disord. 2020;13:1756286420917830. doi: 10.1177/1756286420917830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Y, Li M, Wang M, Zhou Y, Chang J, Xian Y, Wang D, Mao L, Jin H, Hu B. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020;5:279–284. doi: 10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19: A systematic review. J Neurol Sci. 2020;413:116832. doi: 10.1016/j.jns.2020.116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leitinger M, Beniczky S, Rohracher A, Gardella E, Kalss G, Qerama E, Höfler J, Hess Lindberg-Larsen A, Kuchukhidze G, Dobesberger J, Langthaler PB, Trinka E. Salzburg Consensus Criteria for Non-Convulsive Status Epilepticus--approach to clinical application. Epilepsy Behav. 2015;49:158–163. doi: 10.1016/j.yebeh.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 72.Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19:383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jarius S, Bieber N, Haas J, Wildemann B. MOG encephalomyelitis after vaccination against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2): case report and comprehensive review of the literature. J Neurol. 2022;269:5198–5212. doi: 10.1007/s00415-022-11194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, Ueno M, Sakata H, Kondo K, Myose N, Nakao A, Takeda M, Haro H, Inoue O, Suzuki-Inoue K, Kubokawa K, Ogihara S, Sasaki T, Kinouchi H, Kojin H, Ito M, Onishi H, Shimizu T, Sasaki Y, Enomoto N, Ishihara H, Furuya S, Yamamoto T, Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Galea S, Merchant RM, Lurie N. The Mental Health Consequences of COVID-19 and Physical Distancing: The Need for Prevention and Early Intervention. JAMA Intern Med. 2020;180:817–818. doi: 10.1001/jamainternmed.2020.1562. [DOI] [PubMed] [Google Scholar]
- 76.World Health Organization. 2019 Novel coronavirus (2019-nCoV): strategic preparedness and response plan. [Internet] [accessed 3 February 2020]. Available from: https://www.who.int/docs/default-source/coronaviruse/srp-04022020.pdf?sfvrsn=7ff55ec0_4 .
- 77.Center of Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19), stress and Coping. [Internet] [accessed 10 May 2020]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/managing-stress-anxiety.html .
- 78.Jakovljevic M, Bjedov S, Jaksic N, Jakovljevic I. COVID-19 Pandemia and Public and Global Mental Health from the Perspective of Global Health Securit. Psychiatr Danub. 2020;32:6–14. doi: 10.24869/psyd.2020.6. [DOI] [PubMed] [Google Scholar]
- 79.Wu KK, Chan SK, Ma TM. Posttraumatic stress, anxiety, and depression in survivors of severe acute respiratory syndrome (SARS) J Trauma Stress. 2005;18:39–42. doi: 10.1002/jts.20004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mak IW, Chu CM, Pan PC, Yiu MG, Chan VL. Long-term psychiatric morbidities among SARS survivors. Gen Hosp Psychiatry. 2009;31:318–326. doi: 10.1016/j.genhosppsych.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wing YK, Ho SMY. Mental health of patients infected with SARS. In: Chan JCK, Wong VCWT, editors. Challenges of Severe Acute Respiratory Syndrome. Hong Kong: Elsevier (Singapore) Pte Ltd; 2006. p590. [Google Scholar]
- 82.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, Dequanter D, Blecic S, El Afia F, Distinguin L, Chekkoury-Idrissi Y, Hans S, Delgado IL, Calvo-Henriquez C, Lavigne P, Falanga C, Barillari MR, Cammaroto G, Khalife M, Leich P, Souchay C, Rossi C, Journe F, Hsieh J, Edjlali M, Carlier R, Ris L, Lovato A, De Filippis C, Coppee F, Fakhry N, Ayad T, Saussez S. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, Rusconi S, Gervasoni C, Ridolfo AL, Rizzardini G, Antinori S, Galli M. Self-reported Olfactory and Taste Disorders in Patients With Severe Acute Respiratory Coronavirus 2 Infection: A Cross-sectional Study. Clin Infect Dis. 2020;71:889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaye R, Chang CWD, Kazahaya K, Brereton J, Denneny JC 3rd. COVID-19 Anosmia Reporting Tool: Initial Findings. Otolaryngol Head Neck Surg. 2020;163:132–134. doi: 10.1177/0194599820922992. [DOI] [PubMed] [Google Scholar]
- 85.Moein ST, Hashemian SM, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 2020;10:944–950. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu AY, Tu R, Shao X, Pan A, Zhou K, Huang J. A comprehensive Chinese experience against SARS-CoV-2 in ophthalmology. Eye Vis (Lond) 2020;7:19. doi: 10.1186/s40662-020-00187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Seah I, Agrawal R. Can the Coronavirus Disease 2019 (COVID-19) Affect the Eyes? Ocul Immunol Inflamm. 2020;28:391–395. doi: 10.1080/09273948.2020.1738501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Holappa M, Vapaatalo H, Vaajanen A. Many Faces of Renin-angiotensin System - Focus on Eye. Open Ophthalmol J. 2017;11:122–142. doi: 10.2174/1874364101711010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu P, Duan F, Luo C, Liu Q, Qu X, Liang L, Wu K. Characteristics of Ocular Findings of Patients With Coronavirus Disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138:575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lescure FX, Bouadma L, Nguyen D, Parisey M, Wicky PH, Behillil S, Gaymard A, Bouscambert-Duchamp M, Donati F, Le Hingrat Q, Enouf V, Houhou-Fidouh N, Valette M, Mailles A, Lucet JC, Mentre F, Duval X, Descamps D, Malvy D, Timsit JF, Lina B, van-der-Werf S, Yazdanpanah Y. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. 2020;20:697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cheema M, Aghazadeh H, Nazarali S, Ting A, Hodges J, McFarlane A, Kanji JN, Zelyas N, Damji KF, Solarte C. Keratoconjunctivitis as the initial medical presentation of the novel coronavirus disease 2019 (COVID-19) Can J Ophthalmol. 2020;55:e125–e129. doi: 10.1016/j.jcjo.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mustafa MWM. Audiological profile of asymptomatic Covid-19 PCR-positive cases. Am J Otolaryngol. 2020;41:102483. doi: 10.1016/j.amjoto.2020.102483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fidan V. New type of corona virus induced acute otitis media in adult. Am J Otolaryngol. 2020;41:102487. doi: 10.1016/j.amjoto.2020.102487. [DOI] [PMC free article] [PubMed] [Google Scholar]