Abstract
Sepsis-associated encephalopathy (SAE), a major cerebral complication of sepsis, occurs in 70% of patients admitted to the intensive care unit (ICU). This condition can cause serious impairment of consciousness and is associated with a high mortality rate. Thus far, several experimental screenings and radiological techniques (e.g., electroencephalography) have been used for the non-invasive assessment of the structure and function of the brain in patients with SAE. Nevertheless, the pathogenesis of SAE is complicated and remains unclear. In the present article, we reviewed the currently available literature on the epidemiology, clinical manifestations, pathology, diagnosis, and management of SAE. However, currently, there is no ideal pharmacological treatment for SAE. Treatment targeting mitochondrial dysfunction may be useful in the management of SAE.
Keywords: Sepsis-associated encephalopathy, Long-term cognitive dysfunction, Cerebral microvasculature damage
Introduction
Sepsis-associated encephalopathy (SAE) is a diffuse cerebral dysfunction caused by a systemic inflammatory response to sepsis. This complication is characterized by abnormal brain structure, cerebral hemorrhage, and cerebral embolism. The clinical manifestation of SAE ranges from mild delirium to severe coma and is accompanied by brain dysfunction (e.g., behavior, cognition, consciousness) and perception changes. SAE is linked to an increased mortality rate and can cause sequelae, such as long-term cognitive dysfunction, in patients with sepsis [1]. The pathogenesis of SAE is complicated and remains unclear so far. Furthermore, SAE may occur at any stage of sepsis, even before the occurrence of other symptoms. Currently, there is no established specific therapy or pharmacological treatment for SAE. The lack of effective treatments seriously affects the prognosis of patients with sepsis. Therefore, in this article, we reviewed the main characteristics, pathophysiology, therapeutic options, and prognosis of SAE.
Epidemiology
SAE is the most common cerebral dysfunction in patients admitted to the intensive care unit (ICU), with an incidence of 9%–71%. The incidence of SAE is even higher in the presence of bacteremia and multiple organ failure [1]. Notably, >70% of patients with bacteremia develop severe neurological symptoms, ranging from lethargy to coma. In addition, examination through electroencephalography (EEG) reveals abnormalities in >80% of patients with bacteremia [2]. The Acute Physiology and Chronic Health Evaluation II (APACHE II) score is higher in patients with SAE than those without SAE. In contrast, the Glasgow Coma Scale score is lower in patients with SAE than those without SAE [3]. In addition, the duration of mechanical ventilation and ICU stay is longer in patients with SAE than those without SAE. It has been demonstrated that encephalopathy increases the mortality rate among patients with sepsis; this effect is mainly associated with the clinical and electrophysiological severity of SAE. Nevertheless, the mechanisms involved in this process have not been elucidated yet.
Clinical Manifestation
Although the clinical manifestations of SAE are diverse, they lack specificity. Acute change in mental status (e.g., inattention, disorientation, agitation, somnolence, stupor, coma) is the first manifestation of SAE. Other clinical features of SAE include roving eye movements, asterixis, tremor, multifocal myoclonus, restlessness, tachypnea, seizures, paratonic rigidity, extensor plantar responses, and flexor or extensor posturing [4,5]. More importantly, SAE can cause long-term cognitive dysfunction, which affects the following: working memory, attention, and task switching (the cortical connection between the frontal and parietal lobes); language learning and memory (hippocampus and adjacent hippocampus back); and fluent speech and language (prefrontal cortex) [6]. Approximately, 45% and 10% of the patients with sepsis continue to have long-term cognitive dysfunction 1 year and 3 years after discharge, respectively. Importantly, impairment of cognitive functions (e.g., inattention and memory decline) may persist even 6 years after recovery [6,7]. It has been shown that SAE increases the risk of suicide within 2 years after recovery [8]. These psychological and cognitive disorders drastically impact the quality of life and daily activities of patients with SAE.
Pathogenesis
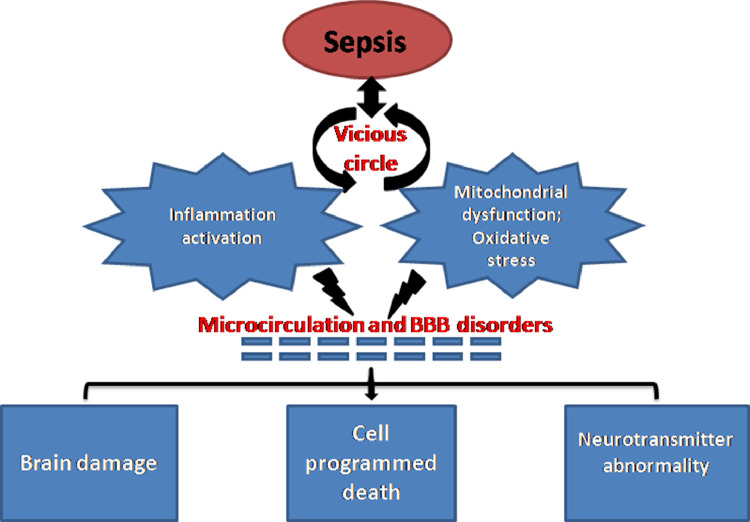
SAE is an inflammatory response to sepsis-induced systemic infection. During the inflammatory cascade, impaired fibrinolysis activates the pro-coagulant pathways, thereby resulting in excessive fibrin deposition. This reduces the activity of activated protein C, anti-thrombin, and tissue factor inhibitor, leading to micro-circulation thrombosis through impairment of anti-coagulant pathways and tissue hypo-perfusion. Arterial hypotension, reduced red blood cell deformability, and oxidative stress-induced mitochondrial damage also contribute to the impairment of tissue oxygenation [9,10]. The neuroinflammation and oxidative stress lead to tissue hypoperfusion through loss of cerebral endothelial barrier integrity, resulting in cell programmed death and end organ damage. It has been established that these factors are associated with acute septic encephalopathy and long-term neurocognitive sequelae [11]. Potential pathophysiologic mechanisms leading to SAE are summarized in Fig. 1.
Fig. 1.
Potential pathophysiologic mechanisms leading to SAE. Neuroinflammation, mitochondrial dysfunction, and oxidative stress damage the cerebral microcirculation and BBB, causing neurotransmitter abnormality, cell programmed death, and culminating in brain damage and dysfunction. BBB: Blood–brain barrier; SAE: Sepsis-associated encephalopathy.
Activation of inflammation
Tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) are the most important inflammatory factors in the early stages of sepsis. TNF-α can cause neutrophil infiltration in brain tissue and nerve cell apoptosis, brain tissue edema, and dysfunction of the blood-brain-barrier (BBB). These effects severely impair dopamine, norepinephrine, and serotonergic neurotransmission in the central nervous system (CNS), leading to cognitive decline [12]. IL-6 also plays a vital role in SAE. In glial cells, IL-6 increases the expression of cyclooxygenase 2 (COX-2) and prostaglandin synthesis, particularly associated with the hypothalamic-pituitary-adrenal axis and prostaglandin E2 phase. These changes eventually lead to fever and changes in behavior [13]. High mobility group protein B1 (HMGB1) is a pro-inflammatory factor with three redox subunits, namely disulfide, thiol, and sulfonyl [14]. Disulfide HMGB1 inhibits the cell surface receptor complex myeloid differentiation 2 (MD2)-toll-like receptor 4 (TLR4), promotes the release of TNF-α and IL-6, and further aggravates the inflammatory response [15]. HMGB1 also plays a vital role in the late stage of sepsis. Sepsis can increase the level of HMGB1 in the blood circulation of patients, which is closely related to mortality in hospitals [16]. Studies have shown that intraperitoneal injection of lipopolysaccharide-activated microglia was accompanied by an expression of cellular TLRs in host cells. This may induce systemic inflammation through the upregulation of pro-inflammatory factors, such as TNF-α and IL-1β. In turn, this process promotes the release of blood mediators, such as prostaglandins, leukotrienes, platelet-activating factor, and phosphatase A2. These mediators induced leakage by damaging the endothelial cells of capillaries and small blood vessels. They also activate the generation of nitric oxygen from macrophages and neutrophils, causing vasodilation and reducing blood pressure. These alterations lead to hypoperfusion in the brain, eventually causing SAE [17,18].
Mitochondrial dysfunction and oxidative stress
Mitochondria is the main site of oxidative phosphorylation, where energy is produced for the metabolism of cells. Studies have shown that sepsis can destroy mitochondria, resulting in damage to the respiratory chain, reduction in energy production, and excessive production of free radicals. A study showed that reduced mitochondrial oxidative phosphorylation and overproduction of reactive oxygen species (ROS) were detected in septic cells. Most importantly, ROS-induced oxidative stress causes mitochondrial dysfunction in neurons by altering the membrane potential of mitochondria and nitration of mitochondrial proteins [19]. These pathological changes limited the energy production and induced apoptosis in the cells, which ultimately affected the recovery of patients with sepsis [20]. Mitochondrial dysfunction can also promote the occurrence of septic shock and even cause multiple organ dysfunction syndromes, forming a vicious circle [21]. Therefore, mitochondrial dysfunction may play an important role in the pathogenesis of SAE.
Following the occurrence of SAE, NADPH oxidase 2 (NOX2) can mediate the production of ROS, increase hippocampal oxidative stress, and impair cognitive function [22]. SAE also causes an imbalance in the cascade of lipid peroxidation reactions and reverses the cellular protective effects of antioxidants in the cerebrum, blood vessels, and functional tissues [23]. Wu et al. [24] found that increased production of ROS in the hippocampus further caused neuronal apoptosis and increased inflammation in an SAE model. The inflammatory response and ROS promote each other, causing lipid peroxidation and oxidative damage to proteins and DNA, eventually resulting in cell damage and death [25].
Cerebral microcirculation and BBB disorders
The integrity of cerebral microcirculation and the BBB is essential for maintaining the normal function of the brain. Decreased microcirculation and damaged cerebral microvasculature are key features of cerebral microcirculatory abnormalities, observed during both the onset and progression of SAE [26]. A growing body of evidence has shown that abnormal cerebral microcirculatory in sepsis is a major cause of SAE. In an SAE model induced by sheep peritonitis, damaged cerebral vascular endothelial cells triggered dysfunction of brain microcirculation, thereby reducing cerebral perfusion and cerebral oxygen tension [27].
The activation and dysfunction of endothelial cells could affect the microcirculation and integrity of the BBB, playing an important role in the pathogenesis of SAE [28]. Activated endothelial cells release pro-inflammatory mediators and nitric oxide, which interact with surrounding brain cells. This process leads to the occurrence of inflammation in the brain tissue and increases the expression of adhesion molecules and TLR4. Activated lymphocytes and neutrophils adhere to the microvessels of the CNS, causing encephalitis symptoms. In addition, the activation of endothelial cells can cause dysfunction of blood coagulation, further causing brain tissue microcirculation disorders, as well as abnormal nutrient supply and metabolism. This may lead to cerebral ischemia and hemorrhage, which could aggravate brain tissue damage during SAE [29].
The disintegration of the BBB is one of the main causes of sepsis-induced cerebral dysfunction and systemic damage. Normally, the BBB protects brain tissue from various harmful factors and creates a tightly controlled nerve cell microenvironment. The astrocyte end-feet encircling endothelial cells and pericytes maintain the integrity of the BBB. During the pathological process of sepsis, the dysfunction of endothelial cells and destroyed BBB allow neurotoxic factors to enter the brain tissue. Studies have revealed that endotoxemia may result in the destruction of tight junctions of the BBB in endothelial cells and the separation of pericytes in the hippocampus [30]. Neutrophils are essential for the host defense system; however, an excessive number of neutrophils generate toxic inflammatory intermediates, which induce the damage mechanisms of sepsis. SAE can alter neutrophil-integrin interactions as well as neutrophil recruitment and migration, thereby impairing the transendothelial electrical resistance and integrity of the BBB. This pathway is mediated by the zonula occludens-1 (ZO-1) and occludin in tight junction and cadherin junction complexes of the BBB. This process is regulated by the protein kinase C-delta (PKCδ) of the PKC super-family [31]. Moreover, sepsis-induced oxidative stress due to the overexpression of superoxides and hydroperoxides and disrupted the normal functions of electronic transport chains and mitochondrial respiration. These effects induced long-term oxidative damage to endothelial cells and overexpression of matrix metalloproteases, resulting in degradation of the extracellular matrix and alteration of the basement membrane formation in the BBB [32]. Disruption and leakage of the BBB lead to neuronal cell damage and eventually death.
Thus, targeting the intermediate mechanisms involved in cerebral microcirculation disorders and BBB disruption may lead to the development of innovative and effective treatment strategies against SAE.
Brain damage and cell programmed death
Magnetic resonance imaging (MRI) of the brain in patients with sepsis shows atrophy and abnormal low-density shadows in the peri‑ventricular white matter, accompanied by brain tissue swelling. These findings suggest that SAE can cause direct damage to brain tissue [33]. Neuron-specific enolase (NSE) is an enolase involved in the cytoplasmic glycolysis pathway in neurons and neuroendocrine cells. Following damage to brain tissue, NSE is released into the blood and cerebrospinal fluid, and its concentration increases significantly [15]. The S100 proteins, mainly including S100α and S100β, refer to a type of calcium-binding proteins in the nervous system with low molecular weight. At the physiological concentration, S100β protein exerts a neurotrophic effect; in contrast, at high concentrations, S100β protein is neurotoxic. This protein can increase the cerebral susceptibility to ischemia and hypoxia and trigger neuronal apoptosis. Studies have shown that the concentrations of NSE and S100β were significantly increased in the serum of patients with SAE vs. those without SAE (96.6 ± 8.9 mg/L vs. 4.0 ± 1.3 mg/L, respectively, P < 0.001; and 10.5 ± 2.4 mg/L vs. 0.9 ± 0.1 mg/L, respectively, P < 0.001). However, the sensitivity and specificity of S100β in the diagnosis and prognosis of SAE were higher than those of NSE [34,35].
Brain cells are susceptible to oxidative stress damage. Damage caused by oxidative stress was observed in multiple brain areas of septic rats, particularly in the hippocampus and cortex [29]. Patients with SAE often develop long-term cognitive dysfunction during the recovery period, indicating that SAE may also indirectly trigger the destruction of brain cells. In addition, studies have shown that sepsis caused neurodegenerative diseases in animals. Similar to Alzheimer's disease, the effects included an increase in the levels of amyloid-beta peptide in the hippocampus [36]. The above studies suggested that the pathogenesis of SAE is closely associated with both direct and indirect brain damage.
Increased neuronal apoptosis had been found in the hippocampus during SAE, causing severe neurocognitive impairments [37]. In a cecal ligation and puncture (CLP) sepsis rat model, increased levels of Bcl-2-associated X (Bax) and decreased levels of Bcl2 were observed in hippocampal and cortical cells [38]. The phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) pathway plays an important role in sepsis-induced neuronal apoptosis. AKT phosphorylation alleviated mitochondrial damage, oxidative stress, and neuroinflammation and attenuated SAE-induced neuronal apoptosis by increasing the expression of Bcl-2 and suppressing that of Bax [39]. Omi/high-temperature requirement A2 (Omi/HtrA2) is a proapoptotic serine protease involved in caspase-dependent and caspase-independent cell apoptosis. Studies have found that UCF-101, a specific inhibitor of Omi/HtrA2, has neuroprotective effects by reducing cerebral oxidative injury and cognitive impairment in septic rats. This evidence revealed the involvement of a mitochondria-dependent apoptotic pathway [40]. Activation of the P38-mitogen-activated protein kinase (P38-MAPK) signaling pathway was observed in sepsis-induced cerebral damage. A P38-MAPK inhibitor diminished the CLP-induced apoptosis in cortical and hippocampal cells, indicating its key role in this process [38].
Autophagy also plays an important role in SAE-induced cell programmed death. The intracellular autophagic flux was enhanced through the increased expression of autophagic markers and microtubule-associated proteins 1A/1B-light chain 3B (LC3B) and decreased expression of sequestosome 1/P62 (SQSTM1/P62) in SAE [41]. Similarly, hippocampal mammalian target of rapamycin (mTOR) activation was associated with sepsis-induced cognitive dysfunction in a CLP model [42]. Recently, a study demonstrated that increased activation of caspases was associated with an elevated Bax/Bcl2 ratio, suggesting cross-talk between the autophagy and apoptosis pathways [43].
Studies have shown that NLR pyrin domain-containing 3/caspase-1 (NLRP3/CASP1)-mediated pyroptosis induced the maturation of inflammatory cells and cognitive dysfunction in mice with SAE. Club cell protein 16 (CC16), a secretory protein, alleviated CLP-induced cerebral damage by inhibiting cellular pyroptosis and apoptosis and activating autophagy via the P38-MAPK signaling pathway. These observations indicated that the P38-MAPK signaling pathway is essential in sepsis-induced cell programmed death [38,44].
Neurotransmitter abnormality
Studies have shown that the levels of brain-derived neurotrophic factor (BDNF) in hippocampal tissue of septic animals were significantly decreased. This was accompanied by a significant decrease in cognitive function, suggesting that BDNF plays a vital role in the pathogenesis of SAE [45]. Jeremias et al. [45] used donepezil, a long-acting and reversible cholinesterase inhibitor, to increase cholinergic transmission. They found that donepezil could significantly improve the memory and recognition of mice with sepsis-induced by CLP. While the cholinergic transmission was reduced in vesicular acetylcholine transporter-knockdown (VACht-KD) gene mice, aggravation of the above symptoms of sepsis was noted. This suggested that damage to the cholinergic nerve pathway may be related to the pathogenesis of sepsis. Li et al. [46] found that the levels of serotonin (5-hydroxytryptamine [5-HT]) were significantly increased in the plasma of septic mice, which in turn increased the permeability of cerebral vascular endothelial cells. It has been shown that paroxetine, which is used to inhibit the 5-HT transporter and reduce its uptake rate, reduced cerebral microvascular perfusion and improved microvascular dysfunction. Hence, the dysfunction of neurotransmitters plays an important role in the pathogenesis of SAE. Nevertheless, the mechanisms involved in this process remain unclear. Thus, further investigation in this area is warranted.
Septic shock leads to severe hypoperfusion, causing an imbalance in the oxygen requirement in the brain, cellular metabolism, and energy production. It also impairs the function of the microvasculature and induces cerebral hypoxia, thereby culminating in neuronal cell death and long-term cognitive dysfunction. Numerous studies are focusing on the interplay between cell programmed death, neuroinflammation, oxidative stress, BBB dysfunction, and neurotransmitter pathways. Nonetheless, there is a lack of comprehensive studies investigating the link between these causative factors and cognition in SAE. The research focused on the P38-MAPK signaling pathway may provide a new therapeutic strategy against SAE.
Diagnosis
SAE is an exclusive diagnosis. Before the diagnosis of SAE, the influence of drugs must be ruled out. Subsequently, other potential causes of encephalopathy, such as primary diseases of the CNS and abnormal brain function caused by dysfunction of other organs (e.g., liver, kidneys, lungs, and heart), should also be excluded. Sedative drugs are often used in patients with sepsis; however, this treatment may mask symptoms, such as nervous system disorders. The diagnosis of SAE requires the identification of brain dysfunction, which mainly depends on the examination of clinical electrophysiological and biochemical indicators. In clinical practice, the Glasgow Coma Scale score, ICU delirium assessment method, and assessment of the adaptability to the ICU environment are often used to determine the mental state of a patient, as well as the course of SAE and its prognosis [33]. In addition, cranial computed tomography (CT), MRI, and EEG are often used as auxiliary tools for the diagnosis of SAE [Table 1]. Although CT and MRI lack specificity, they can rule out brain dysfunction caused by other reasons. Recently, evidence from MRI studies showed heterogeneous patterns of brain injury in patients with acute-stage SAE, including ischemic lesions, white matter hyperintensities, edema, brain atrophy, focal hemorrhages, and changes in brain volume [47], [48], [49], [50], [51].
Table 1.
Diagnostic findings in SAE.
| Author and year | Number of patients | Diagnostic method | Findings |
|---|---|---|---|
| Young et al. 1992 [52] | 62 | EEG | Severity of SAE associated with severity of EEG abnormalities |
| Delta and suppression associated with mortality TWs associated with mortality | |||
| Sharshar et al. 2007 [50] | 9 | MRI | White matter hyperintensities; Ischemic lesions |
| Suchtya et al. 2010 [47] | 64 | CT/MRI | White matter hyperintensities |
| Brain atrophy | |||
| Edema | |||
| Focal hemorrhages | |||
| Polito et al. 2013 [48] | 71 | MRI/EEG | White matter hyperintensities |
| Ischemic lesions | |||
| Malignant EEG pattern associated with chronic leukoencephalopathy and acute brain ischemia | |||
| Sutter et al. 2013 [53] | 105 | EEG | Theta/delta associated with the poor outcome TWs associated with more severe alteration of consciousness and with higher mortality |
| Kurtz et al. 2014 [55] | 154 | cEEG | PEDs persisting for >24 h associated with the poor outcome NCSE associated with poor outcome |
| Orhun et al. 2020 [49] | 93 | MRI | MRI white matter hyperintensities |
| Ischemic lesions | |||
| Brain atrophy (limbic structures) |
CT: Computed tomography; cEEG: Continuous electroencephalography; EEG: Electroencephalogram; MRI: Magnetic resonance imaging; NCSE: Nonconvulsive status epilepticus; PEDs: Periodic epileptiform discharges; SAE: Sepsis-associated encephalopathy; TWs: Triphasic waves.
Compared with CT and MRI, EEG can be used as a sensitive tool for the diagnosis of SAE, particularly in the early stage. The EEG of patients with SAE showed progressive retardation about the level of consciousness through theta, delta, or triphasic waves (TWs), burst suppression, and periodic epileptiform discharges (PEDs) or nonconvulsive status epilepticus (NCSE) [52,53]. The rate of mortality increased with the severity of EEG abnormalities: 36% mortality with evidence of delta wave; 50% mortality with TWs; and 67% mortality with burst suppression. Under continuous EEG monitoring, the incidence of subclinical seizures in the presence of sepsis in patients is <10% [52,54,55].
Some laboratory test indicators in the serum of patients with SAE (e.g., NSE and S100β) can be used as a supplementary standard for the diagnosis of SAE; nevertheless, the specificity of NSE and S100β remains controversial [15]. The differential diagnosis of SAE includes CNS infection due to bacteria, viruses, fungi or parasitic meningitis, epidural or subdural empyema, brain abscess, immune-related encephalitis, alcohol or drug poisoning, status epilepticus without convulsions, Wernicke encephalopathy, reversible posterior encephalopathy syndrome, serotonin syndrome, and malignant catatonia [17,38].
Clinical Treatments
Currently, there is no specific etiological treatment, symptomatic treatment, or supportive therapy for SAE. The discovery and elimination of reversible pathogenic factors (e.g., hypoxemia, hypercapnia, hypotension, hyperthermia or hypothermia, liver renal dysfunction, and metabolic or electrolyte disturbances) may be a reasonable therapeutic strategy against SAE. In the early stage of SAE, the source of infection should be promptly determined, and appropriate therapy should be administered. Delirium should be actively treated, and supportive treatment is also necessary [56]. Clinical studies have revealed that insulin therapy exerts a neuroprotective effect in SAE [17]. In addition, an SAE animal model study found that inhibition of the induction of conductive nitric oxide synthase could prevent lipopolysaccharide-induced apoptosis in neuronal cells [13]. A large, multicenter, randomized clinical trial showed that treatment with a typical anti-psychotic (haloperidol) or an atypical anti-psychotic (ziprasidone) did not shorten the duration of delirium or coma in patients compared with placebo. There were also no significant differences in mortality, ICU stay, or hospital stay [57]. Compared with lorazepam, dexmedetomidine showed neuroprotective effects in patients with sepsis, namely inhibition of neuronal apoptosis, reduction in the sepsis-associated inflammatory response, and more delirium-free days [58]. Treatment with a combination of anti-inflammatory and anti-oxidant factors may reduce sepsis-associated pathological changes in the brain by blocking the P38-MAPK signaling pathway. Additionally, the use of the PKCδ inhibitor alleviated sepsis-induced damage to the BBB. Hence, it may be useful in attenuating sepsis-induced degeneration of the BBB [31]. UCF-101, a specific inhibitor of Omi/HtrA2, exerted neuroprotective effects on cerebral oxidative injury and cognitive impairment in septic rats, revealing the involvement of a mitochondria-dependent anti-apoptotic pathway in SAE [40]. Although numerous potential therapies have been tested at the experimental level, there are no specific treatment strategies available thus far. In addition, further clinical studies with larger sample sizes are warranted to verify the clinical benefits of such treatments.
Conclusion
SAE is the most common brain disease in the ICU, and it is characterized by various clinical manifestations, lack of special diagnostic and treatment methods, and unclear pathogenesis. Therefore, prompt diagnosis (through careful history inquiry, physical examination, and auxiliary examinations) and treatment of SAE are essential. Although numerous studies have investigated the pathogenesis and strategies for the prevention and treatment of SAE, this condition remains a serious clinical concern. Neuroinflammation, mitochondrial dysfunction, cerebral microcirculation and BBB disorders, neuronal cell programmed death, and neurotransmitter abnormalities have been associated with sepsis. Therefore, clinical research should focus on discovering targets and therapies for these conditions. Moreover, rather than aiming at the resolution of symptoms, targeting the vicious cycle of interlinked mechanisms during SAE may be a novel effective therapeutic and prophylactic strategy against SAE. However, additional large-scale clinical research is warranted to examine the clinical benefits of this approach. An in-depth study of the pathogenesis of SAE may provide useful information for the diagnosis and treatment of this condition.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
The work was supported by the National Natural Science Foundation of China (Grant Number: 82072209).
Managing Editor: Jingling Bao
References
- 1.Ziaja M. Septic encephalopathy. Curr Neurol Neurosci Rep. 2013;13(10):383. doi: 10.1007/s11910-013-0383-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azabou E., Magalhaes E., Braconnier A., Yahiaoui L., Moneger G., Heming N., et al. Early standard electroencephalogram abnormalities predict mortality in septic intensive care unit patients. PLoS ONE. 2015;10(10) doi: 10.1371/journal.pone.0139969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eidelman L.A., Putterman D., Putterman C., Sprung C.L. The spectrum of septic encephalopathy. Definitions, etiologies, and mortalities. JAMA. 1996;275(6):470–473. doi: 10.1001/jama.275.6.470. [DOI] [PubMed] [Google Scholar]
- 4.Hocker S.E., Wijdicks E.F. Neurologic complications of sepsis. Continuum (Minneap Minn) 2014;20:598–613. doi: 10.1212/01.CON.0000450968.53581.ff. 3 Neurology of Systemic Disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molnár L., Fülesdi B., Németh N., Molnár C. Sepsis-associated encephalopathy: A review of literature. Neurol India. 2018;66(2):352–361. doi: 10.4103/0028-3886.227299. [DOI] [PubMed] [Google Scholar]
- 6.Widmann C.N., Heneka M.T. Long-term cerebral consequences of sepsis. Lancet Neurol. 2014;13(6):630–636. doi: 10.1016/S1474-4422(14)70017-1. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Q.H., Sheng Z.Y., Yao Y.M. Septic encephalopathy: When cytokines interact with acetylcholine in the brain. Mil Med Res. 2014;1:20. doi: 10.1186/2054-9369-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lund-Sørensen H., Benros M.E., Madsen T., Sørensen H.J., Eaton W.W., Postolache T.T., et al. A nationwide cohort study of the association between hospitalization with infection and risk of death by suicide. JAMA Psychiatry. 2016;73(9):912–919. doi: 10.1001/jamapsychiatry.2016.1594. [DOI] [PubMed] [Google Scholar]
- 9.Angus D.C., van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(9):840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 10.Remick D.G. Pathophysiology of sepsis. Am J Pathol. 2007;170(5):1435–1444. doi: 10.2353/ajpath.2007.060872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semmler A., Hermann S., Mormann F., Weberpals M., Paxian S.A., Okulla T., et al. Sepsis causes neuroinflammation and concomitant decrease of cerebral metabolism. J Neuroinflammation. 2008;5:38. doi: 10.1186/1742-2094-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erbaş O., Taşkıran D. Sepsis-induced changes in behavioral stereotypy in rats; involvement of tumor necrosis factor-alpha, oxidative stress, and dopamine turnover. J Surg Res. 2014;186(1):262–268. doi: 10.1016/j.jss.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Chaudhry N., Duggal A.K. Sepsis associated encephalopathy. Adv Med. 2014 doi: 10.1155/2014/762320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang H., Wang H., Chavan S.S., Andersson U. High Mobility Group Box Protein 1 (HMGB1): The prototypical endogenous danger molecule. Mol Med. 2015;21(1):S6–S12. doi: 10.2119/molmed.2015.00087. Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu A.A., Fenton K., Weinstein S., Carpenter J., Dalton H., Bell M.J. Neurological injury markers in children with septic shock. Pediatr Crit Care Med. 2008;9(3):245–251. doi: 10.1097/PCC.0b013e3181727b22. [DOI] [PubMed] [Google Scholar]
- 16.Valdés-Ferrer S.I., Papoin J., Dancho M.E., Olofsson P.S., Li J., Lipton J.M., et al. HMGB1 mediates anemia of inflammation in murine sepsis survivors. Mol Med. 2016;21(1):951–958. doi: 10.2119/molmed.2015.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sweis R., Ortiz J., Biller J. Neurology of sepsis. Curr Neurol Neurosci Rep. 2016;16(3):21. doi: 10.1007/s11910-016-0623-z. [DOI] [PubMed] [Google Scholar]
- 18.Tang Y., Le W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol. 2016;53(2):1181–1194. doi: 10.1007/s12035-014-9070-5. [DOI] [PubMed] [Google Scholar]
- 19.Exline M.C., Crouser E.D. Mitochondrial mechanisms of sepsis-induced organ failure. Front Biosci. 2008;13:5030–5041. doi: 10.2741/3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arulkumaran N., Deutschman C.S., Pinsky M.R., Zuckerbraun B., Schumacker P.T., Gomez H., et al. Mitochondrial function in sepsis. Shock. 2016;45(3):271–281. doi: 10.1097/SHK.0000000000000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai F., Zhang Y., Xie D.P., Mai S.T., Weng Y.N., Du J.D., et al. A systematic review of rhubarb (a traditional Chinese medicine) used for the treatment of experimental sepsis. Evid Based Complement Alternat Med. 2015 doi: 10.1155/2015/131283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji M.H., Qiu L.L., Tang H., Ju L.S., Sun X.R., Zhang H., et al. Sepsis-induced selective parvalbumin interneuron phenotype loss and cognitive impairments may be mediated by NADPH oxidase 2 activation in mice. J Neuroinflammation. 2015;12:182. doi: 10.1186/s12974-015-0401-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Towner R.A., Smith N., Saunders D., Lupu F., Silasi-Mansat R., West M., et al. In vivo detection of free radicals using molecular MRI and immuno-spin trapping in a mouse model for amyotrophic lateral sclerosis. Free Radic Biol Med. 2013;63:351–360. doi: 10.1016/j.freeradbiomed.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Wu J., Zhang M., Hao S., Jia M., Ji M., Qiu L., et al. Mitochondria-targeted peptide reverses mitochondrial dysfunction and cognitive deficits in sepsis-associated encephalopathy. Mol Neurobiol. 2015;52(1):783–791. doi: 10.1007/s12035-014-8918-z. [DOI] [PubMed] [Google Scholar]
- 25.Erbaş O., Ergenoglu A.M., Akdemir A., Yeniel A.Ö., Taskiran D. Comparison of melatonin and oxytocin in the prevention of critical illness polyneuropathy in rats with experimentally induced sepsis. J Surg Res. 2013;183(1):313–320. doi: 10.1016/j.jss.2012.11.043. [DOI] [PubMed] [Google Scholar]
- 26.Honig A., Eliahou R., Auriel E. Confined anterior cerebral artery infarction manifesting as isolated unilateral axial weakness. J Neurol Sci. 2017;373:18–20. doi: 10.1016/j.jns.2016.11.061. [DOI] [PubMed] [Google Scholar]
- 27.Taccone F.S., Su F., De Deyne C., Abdellhai A., Pierrakos C., He X., et al. Sepsis is associated with altered cerebral microcirculation and tissue hypoxia in experimental peritonitis. Crit Care Med. 2014;42(2):e114–e122. doi: 10.1097/CCM.0b013e3182a641b8. [DOI] [PubMed] [Google Scholar]
- 28.Nwafor D.C., Brichacek A.L., Mohammad A.S., Griffith J., Lucke-Wold B.P., Benkovic S.A., et al. Targeting the blood-brain barrier to prevent sepsis-associated cognitive impairment. J Cent Nerv Syst Dis. 2019;11 doi: 10.1177/1179573519840652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adam N., Kandelman S., Mantz J., Chrétien F., Sharshar T. Sepsis-induced brain dysfunction. Expert Rev Anti Infect Ther. 2013;11(2):211–221. doi: 10.1586/eri.12.159. [DOI] [PubMed] [Google Scholar]
- 30.Nishioku T., Dohgu S., Takata F., Eto T., Ishikawa N., Kodama K.B., et al. Detachment of brain pericytes from the basal lamina is involved in disruption of the blood-brain barrier caused by lipopolysaccharide-induced sepsis in mice. Cell Mol Neurobiol. 2009;29(3):309–316. doi: 10.1007/s10571-008-9322-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang Y., Soroush F., Sun S., Liverani E., Langston J.C., Yang Q., et al. Protein kinase C-delta inhibition protects blood-brain barrier from sepsis-induced vascular damage. J Neuroinflammation. 2018;15(1):309. doi: 10.1186/s12974-018-1342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldim M.P., Danielski L.G., Rodrigues J.F., Joaquim L., Garbossa L., de Oliveira Junior A.N., et al. Oxidative stress in the choroid plexus contributes to blood-cerebrospinal fluid barrier disruption during sepsis development. Microvasc Res. 2019;123:19–24. doi: 10.1016/j.mvr.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Stubbs D.J., Yamamoto A.K., Menon D.K. Imaging in sepsis-associated encephalopathy – insights and opportunities. Nat Rev Neurol. 2013;9(10):551–561. doi: 10.1038/nrneurol.2013.177. [DOI] [PubMed] [Google Scholar]
- 34.Zenaide P.V., Gusmao-Flores D. Biomarkers in septic encephalopathy: A systematic review of clinical studies. Rev Bras Ter Intensiva. 2013;25(1):56–62. doi: 10.1590/s0103-507x2013000100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao B., Zhang L.N., Ai Y.H., Liu Z.Y., Huang L. Serum S100β is a better biomarker than neuron-specific enolase for sepsis-associated encephalopathy and determining its prognosis: A prospective and observational study. Neurochem Res. 2014;39(7):1263–1269. doi: 10.1007/s11064-014-1308-0. [DOI] [PubMed] [Google Scholar]
- 36.Schwalm M.T., Pasquali M., Miguel S.P., Dos Santos J.P.A., Vuolo F., Comim C.M., et al. Acute brain inflammation and oxidative damage are related to long-term cognitive deficits and markers of neurodegeneration in sepsis-survivor rats. Mol Neurobiol. 2014;49(1):380–385. doi: 10.1007/s12035-013-8526-3. [DOI] [PubMed] [Google Scholar]
- 37.Comim C.M., Barichello T., Grandgirard D., Dal-Pizzol F., Quevedo J., Leib S.L. Caspase-3 mediates in part hippocampal apoptosis in sepsis. Mol Neurobiol. 2013;47(1):394–398. doi: 10.1007/s12035-012-8354-x. [DOI] [PubMed] [Google Scholar]
- 38.Zhou R., Qu Y., Huang Q., Sun X., Mu D., Li X. Recombinant CC16 regulates inflammation, oxidative stress, apoptosis and autophagy via the inhibition of the p38MAPK signaling pathway in the brain of neonatal rats with sepsis. Brain Res. 2019;1725 doi: 10.1016/j.brainres.2019.146473. [DOI] [PubMed] [Google Scholar]
- 39.Wang G.B., Ni Y.L., Zhou X.P., Zhang W.F. The AKT/mTOR pathway mediates neuronal protective effects of erythropoietin in sepsis. Mol Cell Biochem. 2014;385(1–2):125–132. doi: 10.1007/s11010-013-1821-5. [DOI] [PubMed] [Google Scholar]
- 40.Hu Y., Bi Y., Yao D., Wang P., Li Y. Omi/HtrA2 protease associated cell apoptosis participates in blood-brain barrier dysfunction. Front Mol Neurosci. 2019;12:48. doi: 10.3389/fnmol.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mai C., Qiu L., Zeng Y., Jian H.G. LncRNA Lethe protects sepsis-induced brain injury via regulating autophagy of cortical neurons. Eur Rev Med Pharmacol Sci. 2019;23(11):4858–4864. doi: 10.26355/eurrev_201906_18073. [DOI] [PubMed] [Google Scholar]
- 42.Liu W., Guo J., Mu J., Tian L., Zhou D. Rapamycin protects sepsis-induced cognitive impairment in mouse hippocampus by enhancing autophagy. Cell Mol Neurobiol. 2017;37(7):1195–1205. doi: 10.1007/s10571-016-0449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou R., Sun X., Li Y., Huang Q., Qu Y., Mu D., et al. Low-dose Dexamethasone increases autophagy in cerebral cortical neurons of juvenile rats with sepsis associated encephalopathy. Neuroscience. 2019;419:83–99. doi: 10.1016/j.neuroscience.2019.09.020. [DOI] [PubMed] [Google Scholar]
- 44.Zhou R., Yang X., Li X., Qu Y., Huang Q., Sun X., et al. Recombinant CC16 inhibits NLRP3/caspase-1-induced pyroptosis through p38 MAPK and ERK signaling pathways in the brain of a neonatal rat model with sepsis. J Neuroinflammation. 2019;16(1):239. doi: 10.1186/s12974-019-1651-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeremias IC, Victorino V.J., Barbeiro H.V., Kubo S.A., Prado C.M., Lima T.M., et al. The role of acetylcholine in the inflammatory response in animals surviving sepsis induced by Cecal ligation and puncture. Mol Neurobiol. 2016;53(10):6635–6643. doi: 10.1007/s12035-015-9538-y. [DOI] [PubMed] [Google Scholar]
- 46.Li Y., Hadden C., Cooper A., Ahmed A., Wu H., Lupashin V.V., et al. Sepsis-induced elevation in plasma serotonin facilitates endothelial hyperpermeability. Sci Rep. 2016;6:22747. doi: 10.1038/srep22747. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Suchyta M.R., Jephson A., Hopkins R.O. Neurologic changes during critical illness: Brain imaging findings and neurobehavioral outcomes. Brain Imaging Behav. 2010;4(1):22–34. doi: 10.1007/s11682-009-9082-3. [DOI] [PubMed] [Google Scholar]
- 48.Polito A., Eischwald F., Maho A.L., Polito A., Azabou E., Annane D., et al. Pattern of brain injury in the acute setting of human septic shock. Crit Care. 2013;17(5):R204. doi: 10.1186/cc12899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orhun G., Esen F., Özcan P.E., Sencer S., Bilgiç B., Ulusoy C., et al. Neuroimaging findings in sepsis-induced brain dysfunction: Association with clinical and laboratory findings. Neurocrit Care. 2019;30(1):106–117. doi: 10.1007/s12028-018-0581-1. [DOI] [PubMed] [Google Scholar]
- 50.Sharshar T., Carlier R., Bernard F., Guidoux C., Brouland J.P., Nardi O., et al. Brain lesions in septic shock: A magnetic resonance imaging study. Intensive Care Med. 2007;33(5):798–806. doi: 10.1007/s00134-007-0598-y. [DOI] [PubMed] [Google Scholar]
- 51.Orhun G., Tüzün E., Bilgiç B., Ergin Özcan P., Sencer S., Barburoğlu M., et al. Brain volume changes in patients with acute brain dysfunction due to sepsis. Neurocrit Care. 2020;32(2):459–468. doi: 10.1007/s12028-019-00759-8. [DOI] [PubMed] [Google Scholar]
- 52.Young G.B., Bolton C.F., Archibald Y.M., Austin T.W., Wells G.A. The electroencephalogram in sepsis-associated encephalopathy. J Clin Neurophysiol. 1992;9(1):145–152. doi: 10.1097/00004691-199201000-00016. [DOI] [PubMed] [Google Scholar]
- 53.Sutter R., Stevens R.D., Kaplan P.W. Significance of triphasic waves in patients with acute encephalopathy: A nine-year cohort study. Clin Neurophysiol. 2013;124(10):1952–1958. doi: 10.1016/j.clinph.2013.03.031. [DOI] [PubMed] [Google Scholar]
- 54.Oddo M., Carrera E., Claassen J., Mayer S.A., Hirsch L.J. Continuous electroencephalography in the medical intensive care unit. Crit Care Med. 2009;37(6):2051–2056. doi: 10.1097/CCM.0b013e3181a00604. [DOI] [PubMed] [Google Scholar]
- 55.Kurtz P., Gaspard N., Wahl A.S., Bauer R.M., Hirsch L.J., Wunsch H., et al. Continuous electroencephalography in a surgical intensive care unit. Intensive Care Med. 2014;40(2):228–234. doi: 10.1007/s00134-013-3149-8. [DOI] [PubMed] [Google Scholar]
- 56.Gofton T.E., Young G.B. Sepsis-associated encephalopathy. Nat Rev Neurol. 2012;8(10):557–566. doi: 10.1038/nrneurol.2012.183. [DOI] [PubMed] [Google Scholar]
- 57.Girard T.D., Exline M.C., Carson S.S., Hough C.L., Rock P., Gong M.N., et al. Haloperidol and Ziprasidone for Treatment of Delirium in Critical Illness. N Engl J Med. 2018;379(26):2506–2516. doi: 10.1056/NEJMoa1808217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pandharipande P.P., Sanders R.D., Girard T.D., McGrane S., Thompson J.L., Shintani A.K., et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial. Crit Care. 2010;14(2):R38. doi: 10.1186/cc8916. [DOI] [PMC free article] [PubMed] [Google Scholar]