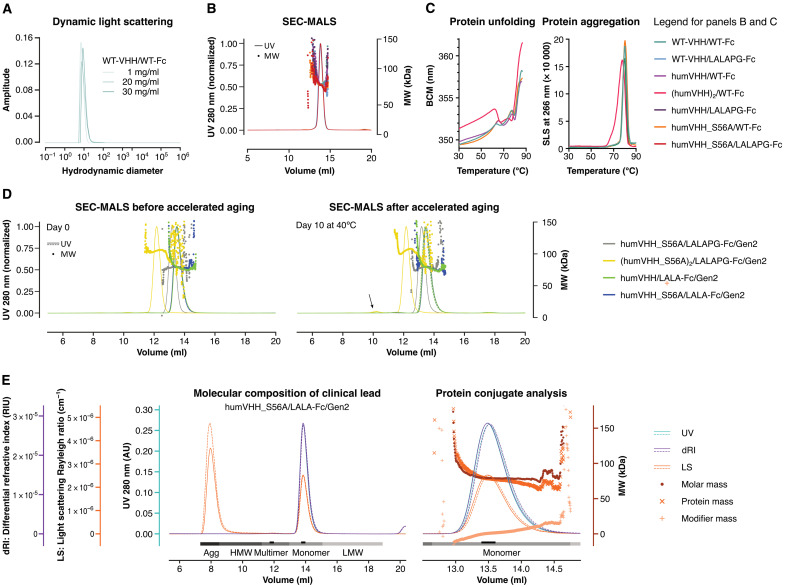
Fig. 4. Biophysical properties of VHH72-Fc constructs.
(A) DLS of WT-VHH/WT-Fc at 25°C is shown, at 1.0, 20, or 30 mg/ml in 25 mM histidine and 125 mM NaCl (pH 6.0). (B) Size exclusion chromatography–multiangle light scattering (SEC-MALS) is shown for samples (2 to 4 mg/ml) of WT-VHH/WT-Fc and variants, in which humanization, affinity-increasing (S56A), and LALAPG mutations were introduced [in 25 mM histidine and 125 mM NaCl (pH6.0)]. UV, ultraviolet 280-nm wavelength light absorption; MW, molar mass of peak fraction determined by light scattering. (C) Thermostability of the indicated VHH72-Fc constructs is shown. Left: Intrinsic tryptophan fluorescence as a measure of protein unfolding during a thermal ramp is expressed as barycentric mean (BCM) of the fluorescence intensity at 300 and 400 nm. Right: Protein aggregation during thermal ramp was measured by static light scattering (SLS) at 260 nm. (D) Ten-day storage at 40°C causes no major changes in SEC-MALS profiles of duplicate VHH72-Fc variant samples (1 mg/ml) in PBS. (E) SEC-MALS profiles of humVHH_S56A/LALA-Fc/Gen2 run in duplicate (solid and dashed lines) are shown. Complete peaks are indicated in grayscale for quantitative analysis; the peak apex is indicated in black for qualitative analysis. Protein conjugate analysis (calculated molar masses shown for a single replicate) was performed on the basis of the differential extinction coefficients and refractive index values of proteins versus conjugated glycan modifiers. AU, arbitrary units.