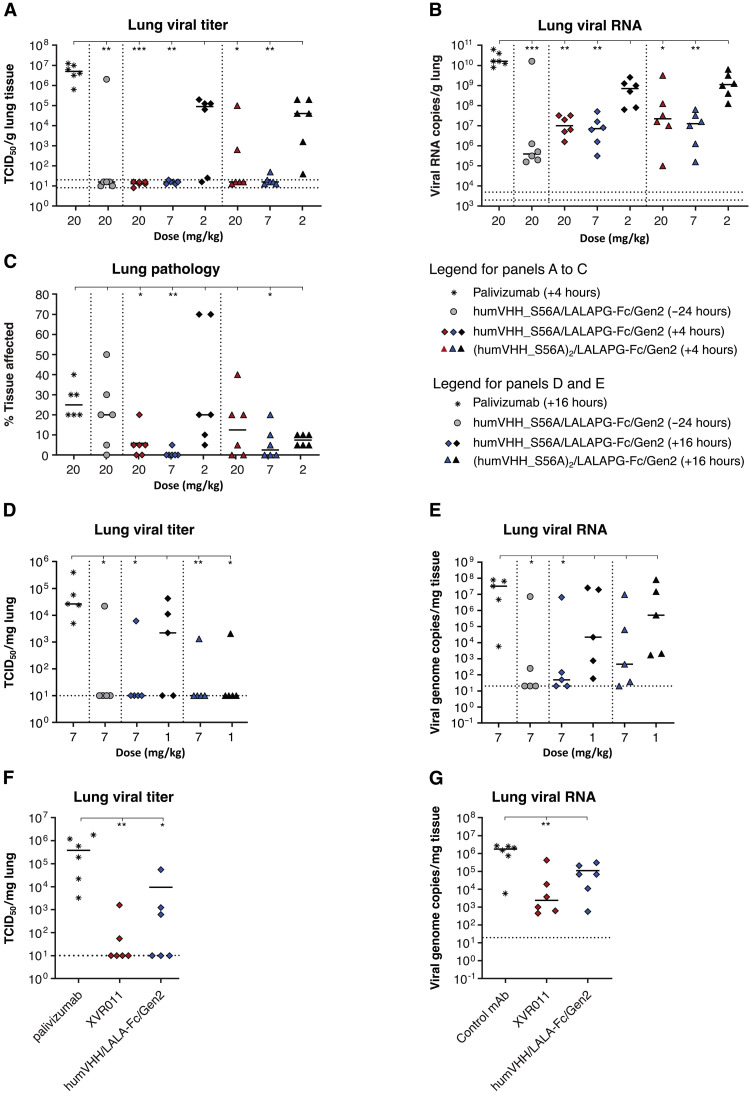
Fig. 5. Therapeutic administration of VHH72-Fc constructs protects hamsters against SARS-CoV-2 challenge.
(A to C) Hamsters were challenged with 1 × 104 PFU of BetaCoV/Munich/BavPat1/2020 and 4 hours later injected intraperitoneally with humVHH_S56A/LALAPG-Fc/Gen2 or (humVHH_S56A)2/LALAPG-Fc/Gen2 (20, 7, or 2 mg/kg). The negative control group received palivizumab (20 mg/kg), and hamsters in a prophylactic control group received humVHH_S56A/LALAPG-Fc/Gen2 (20 mg/kg) 1 day before the challenge. Lung virus loads (A), lung viral RNA copies (B), and gross lung pathology (C) were determined on day 4 after infection. (D and E) Hamsters received an intraperitoneal injection of humVHH_S56A/LALAPG-Fc/Gen2 (7 mg/kg) 1 day before challenge or were treated by intraperitoneal injection of humVHH_S56A/LALAPG-Fc/Gen2 or (humVHH_S56A)2/LALAPG-Fc/Gen2 (1 or 7 mg/kg) 16 hours after infection with 2 × 106 PFU of passage 6 BetaCov/Belgium/GHB-03021/2020. Palivizumab (7 mg/kg) served as a negative control. Infectious virus load (D) and viral RNA (E) were measured in the lungs on day 4 after challenge. (F and G) Hamsters were treated with palivizumab (4 mg/kg), humVHH_S56A/LALA-Fc/Gen2 or humVHH/LALA-Fc/Gen2 by intraperitoneal injection 24 hours after challenge with 2 × 106 PFU of passage 6 BetaCov/Belgium/GHB-03021/2020. Infectious virus (F) and viral RNA (G) were measured in lung tissue on day 4 after infection. Data were analyzed with the Kruskal-Wallis test and Dunn’s multiple comparison test (*P < 0.05, **P < 0.01, and ***P < 0.001). Horizontal bars indicate mean. Dotted horizontal lines indicate lower limit of detection.