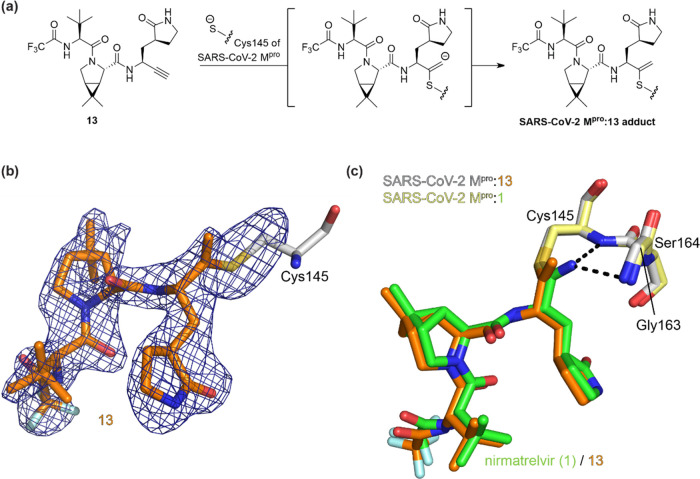
Figure 4.
Crystallographic evidence that the alkyne of the nirmatrelvir derivative 13 reacts covalently with the nucleophilic thiolate of Mpro Cys145. Color code: SARS-CoV-2 Mpro, gray; carbon backbone of 13 in complex with Mpro is in orange; oxygen, red; nitrogen, blue; sulfur, yellow; and fluorine, light blue. (a) Reaction of Mpro with alkyne 13; (b) representative OMIT electron density map (mFo–DFc) contoured to 3σ around Cys145 and 13 in complex with Mpro (PDB ID: 8B2T); (c) superimposition of a view from the Mpro:13 complex structure (PDB ID: 8B2T) with the reported Mpro:nirmatrelvir (1) complex structure (pale yellow: Mpro; green: carbon backbone of 1 in complex with Mpro; PDB ID: 7TE0(22)) showing the interaction of 1 but not 13 with residues forming the oxyanion hole, i.e., Cys145 and Gly143.