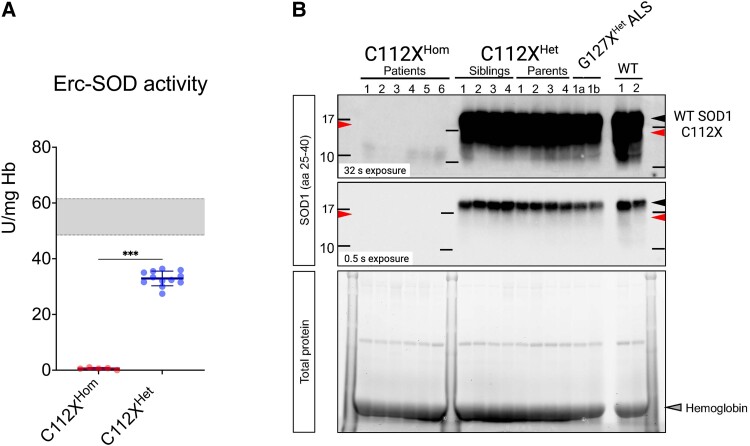
Figure 3.
Absence of SOD activity and SOD1 protein in erythrocyte lysates from homozygous individuals (A) Erythrocyte samples from patients homozygous for the SOD1 c.335dupG, p.C112Wfs*11 (C112XHom) are deficient in SOD1 enzyme activity (0.6 ±0.4 U/mg Hb, P < 0.001 n = 8). In samples from heterozygous carriers (C112XHet), the enzyme activity was approximately halved (32.9 ± 2.6, U/mg Hb, n = 12) compared to WT control samples (reference range 48.5-61.5 U/mg Hb, indicated by the grey box). Data are presented as the mean ± SD and were analyzed using the Mann-Whitney U test. *** P < 0.001. (B) Immunoblotting of erythrocyte lysates using an antibody raised against amino acid residues 25-40 of SOD1 showed positive detection of WT-SOD1 but not p.C112X in heterozygous carriers (n = 8). No SOD1 protein was detected in the samples from homozygous individuals (P1-P6, n = 6) even after extended exposure time (upper panel). A previous study on the lysates of proteasome-inhibited fibroblasts identified a 13-kDa truncated SOD1 protein (arrow C112X) as a product of SOD1 c.335dupG, p.C112X. In addition, WT- SOD1 (black arrow), but not the unstable truncated mutant protein was readily detected in lysates from individuals heterozygous for SOD1 G127X (also known as p.K128Gfs*6, G127XHet), a truncating mutation previously demonstrated to cause adult-onset familial ALS (n = 2). See Supplementary Material for uncropped blots.