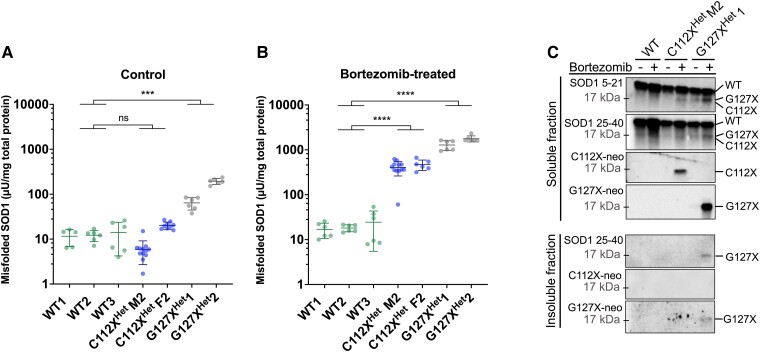
Figure 4.
The truncated SOD1 C112X is efficiently degraded and exhibits low aggregation propensity. (A, B) Plots show levels of soluble misfolded SOD1 detected with an ELISA that specifically detects misfolded, but not natively folded, SOD1 (misELISA) in fibroblast culture extracts from control (WT) (n = 3), C112XHet carriers- (n = 2), or G127XHet ALS patients- (n = 2). All fibroblast lines were derived in house from skin biopsies. (A) Cells from C112XHet individuals show levels of misfolded SOD1 similar to those in control cells under standard culture conditions (P > 0.9999), whereas misSOD1 levels are significantly increased in fibroblasts carrying the previously described ALS-causing truncated variant p.G127X (P = 0.0006). (B) Proteasome inhibition using bortezomib (5 ng/mL) resulted in significantly increased levels of misSOD1, both in cells from C112XHet carriers (P < 0.0001) and G127XHet ALS patients (P < 0.0001) when compared to WT cells. Data are expressed as the mean ± SD of four to twelve technical replicates from 2–3 independent experiments. The means were compared using the Kruskal–Wallis test followed by Dunn’s multiple comparisons test. *P < 0.05, **P < 0.01, ***P < 0.001, **** P < 0.0001, ns, not significant. (C) Western blot on fibroblast extracts. Both WT SOD1 and the truncation variants p.C112X and p.G127X were detected in the soluble fraction using antibodies against the N-terminal sequences of SOD1 (SOD1 5–21 and SOD1 25–40 aa, respectively) and antibodies raised against the p.C112X, and the p.G127X neo-peptides (C112X-neo and G127X-neo, respectively). The p.G127X but no p.C112X was detected in the insoluble fraction.
aa—amino acid, WT—wild-type, C112XHet M2—mother of Patient 2, C112XHet F2—father of Patient 2. The migration pattern varies between the WT and the different truncation variants, as indicated on the right. See Supplementary material for uncropped blots.