Figure 2.
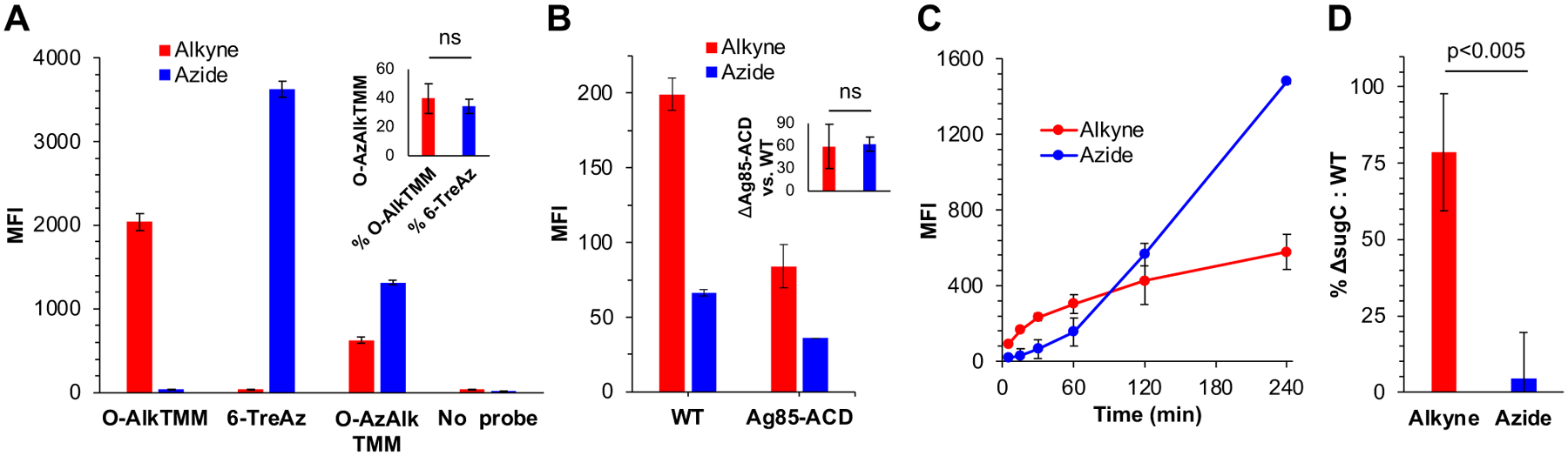
Two-step incorporation of bifunctional reporter O-AzAlkTMM into the M. smegmatis mycomembrane. (A) Labeling of wild-type (WT) M. smegmatis with 50 μM O-AlkTMM, 6-TreAz, or O-AzAlkTMM for 15 min or ~10% generation time. Fluorescence from sequential SPAAC (with DBCO Cy5, to detect azides) and CuAAC (with Carboxyrhodamine 110 Azide, to detect alkynes) was quantitated by flow cytometry. MFI, median fluorescence intensity. Representative data from five independent experiments performed in triplicate are shown. Inset, O-AzAlkTMM labeling as a % of O-AlkTMM or 6-TreAz over the five experiments. (B) Loss of Ag85ACD decreases both alkyne and azide signal from O-AzAlkTMM. WT and Δag85ACD M. smegmatis were labeled with O-AzAlkTMM as in (A), except that alkynes and azides were respectively and individually revealed by CuAAC reaction with complementary azido- or alkynyl-Carboxyrhodamine 110. Representative data from four independent experiments performed in triplicate are shown. Inset, Δag85ACD labeling as a % of wild-type (WT) over the four experiments. (C) Time-dependence of alkyne- and azide-derived labeling from O-AzAlkTMM. Wild-type M. smegmatis was labeled with O-AzAlkTMM as in Fig. 2A and aliquots were taken at the indicated time points for CuAAC as in Fig. 2B. Data from three independent experiments plotted. (D) Azide but not alkyne signal from O-AzAlkTMM is dependent on the presence of SugC. WT and ΔsugC M. smegmatis were labeled as in Fig. 2A and CuAAC performed as in Fig. 2B. Data (from three independent experiments performed in triplicate) for the mutant were normalized to WT and expressed as fold-change. Statistical significance assessed by two-tailed Student’s t test. ns, not significant (p>0.05). Error bars, standard deviation of technical or biological replicates as described above.
