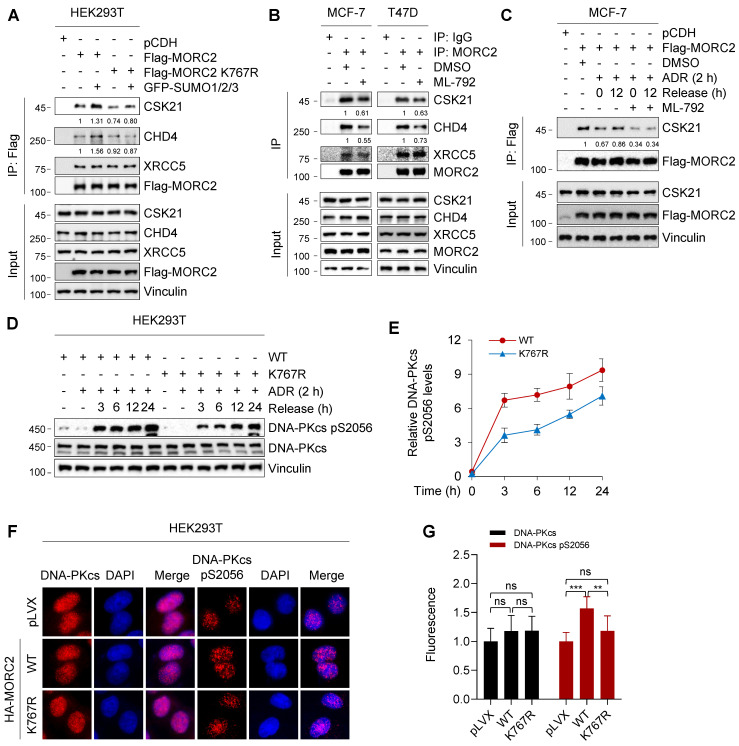
Figure 6.
SUMOylation of MORC2 contributes to DNA repair. (A) HEK293T cells were transfected with WT or K767R MORC2 alone or in combination with GFP-SUMO plasmids for 48 h. Cellular lysates were subjected to IP assays using anti-Flag beads, followed by immunoblotting analysis. (B) MCF-7 and T47D cells were treated with or without 1 μM ML-792 for 24 h. Cellular lysates were subjected to IP assays using anti-MORC2 antibody, followed by immunoblotting analysis. (C) MCF-7 cells stably expressing Flag-MORC2 were treated with 1 μM ADR for 2 h, followed by release for 0 or 12 h, in the presence or absence of 1 μM ML-792. Cellular lysates were harvested for IP assays with anti-Flag beads, followed by immunoblotting analysis. (D-E) HEK293T cells stably expressing WT or K767R MORC2 were treated with 1 μM ADR for 2 h, followed by release for the indicated times, and then subjected to immunoblotting analysis (D). Relative expression levels of DNA-PKcs p2056 (DNA-PKcs pS2056/Vinculin) are shown in E. (F-G) HEK293T cells stably expressing control vector, WT or K767R MORC2 were treated with 1 μM ADR for 2 h, followed by recovery for 24 h, and subjected to immunofluorescent staining with the indicated antibodies (F). Quantification analysis was conducted using ImageJ software, followed by statistical analysis with Student's t test (G).