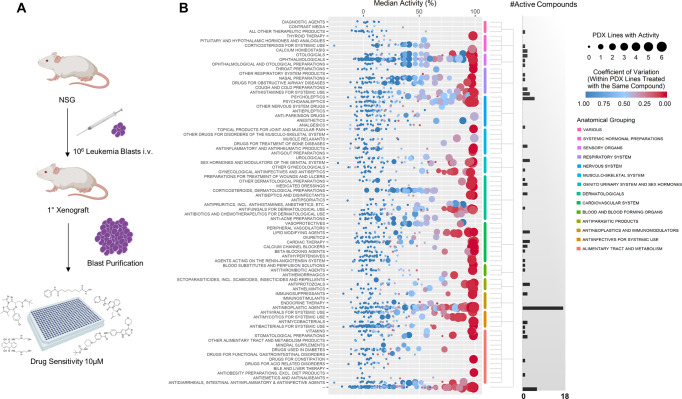
Fig. 1. High throughput screening of infant ALL.
A Study design. Primary leukemia blasts were thawed and injected into immunodeficient mice. Cells were harvested from moribund mice as described in “Methods” and viably stored in liquid nitrogen. Samples were thawed for drug sensitivity studies at a later time point. Figure created with BioRender.com, academic license. B In vitro activity of FDA-approved compounds. Six passaged patient specimens were thawed and cultured in the presence of compounds at a single concentration of 10 µM in duplicate as described in “Methods”. Normalized activity, defined as percent toxicity, was determined for each compound with each patient sample. Each data point corresponds to a single compound, the size of the data point indicates the number of patient samples with 80% or greater activity for that compound and the color indicates the coefficient of variation within the patient samples treated with that compound. Compounds are grouped according to class (see Supplementary Data 1). The number of compounds for each class with greater than 80% activity in five or more patient samples are shown. Source data are provided as a Source data file.