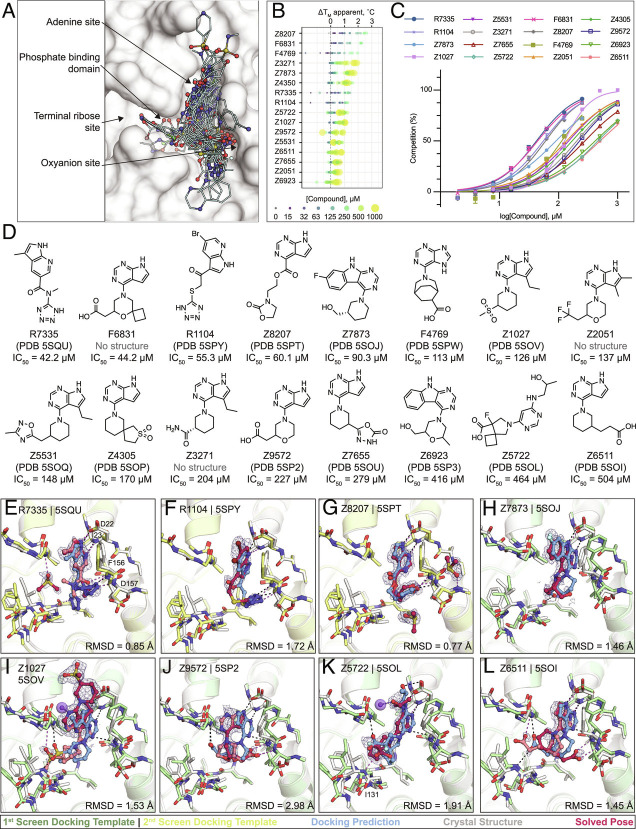
Fig. 5.
Large scale docking targeting the adenosine site of Mac1. A) Binding poses of 47 docking hits confirmed by X-ray crystallography. The ADPr-bound structure of Mac1 (PDB 6W02) is shown with a white surface. B) Thermal upshifts measured by DSF. Data are presented for three technical replicates. C) HTRF-based peptide displacement dose–response curves. Data are presented as the mean ± SEM of at least two repeat measurements. D) 2D structures of docking hits with activity in the HTRF assay. E–L) Crystal structures of Mac1 bound to R7335, R1104, Z8207, Z7873, Z1027, Z9572, Z5722, and Z6511, respectively. The protein structure used in the first docking screen is shown in green, the structure from the second screen is colored yellow. The predicted binding poses are shown in blue. Protein crystal structures are shown in gray and the solved binding poses are shown in red, with alternative ligand conformations colored salmon. Hydrogen bonding interactions between ligands and the Lys11 backbone nitrogen of a symmetry mate are shown with purple dashes/spheres. Hungarian RMSD between the docked and solved ligand poses were calculated with DOCK6. PanDDA event maps are shown for each ligand (contoured at 2 σ).