FIGURE 4.
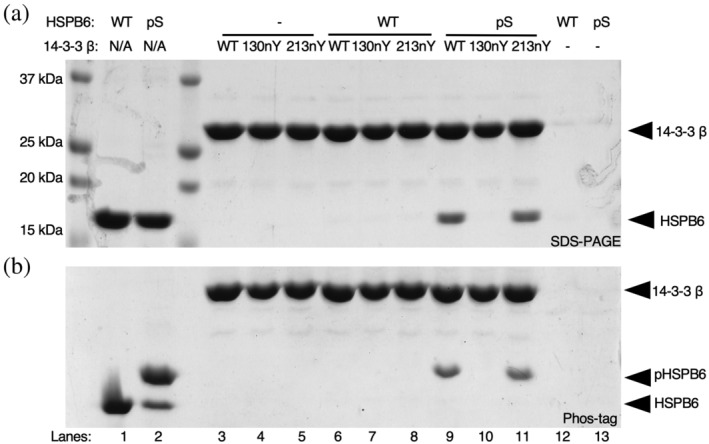
Interaction of nitrated 14‐3‐3 variants with full‐length phosphorylated HSPB6. (a) SDS‐PAGE and (b) Phos‐tag gel analysis of purified HSPB6 (WT) and pHSPB6 (Lanes 1 and 2), as well as pull‐down assays in which biotinylated SUMO‐14‐3‐3 β fusion proteins (WT, 130nY or 213nY) were incubated with no protein (Lanes 3–5), HSPB6 (Lanes 6–8) or pHSPB6 (Lanes 9–11), immobilized onto Streptavidin beads and then eluted by SUMO protease after washing. Phos‐tag electrophoresis has an acrylamide derivative that attenuates the migration of phosphorylated proteins, allowing for easy assessment of phosphorylation status of a protein.
