Abstract
Objective
Subthreshold depression (SD) is a global mental health problem given its high prevalence, comorbidity, functional impairment, and its association with increased service utilization. However, currently little is known about sex differences of SD in cognitive impairment with clinical correlates. This study aims to explore sex differences in subjective cognitive impairment and clinically associated risk factors in Chinese patients with subthreshold depression (SD).
Methods
A total of 126 patients with SD, 40 males and 86 females, aged 18–45 years, were included in this cross-sectional observational study. Their general information, psychological assessments, and psychiatric symptom assessments were collected online. The Patient Health Questionnaire depression-9 (PHQ-9), Generalized Anxiety Disorder-7 (GAD-7), Perceived Deficits Questionnaire-Depression (PDQ-D), and Toronto Alexithymia Scale (TAS-20) with 3 subdomains were used. The obtained scores were analyzed with partial correlation and multiple linear regression analysis models.
Results
Our results showed that females had significantly higher PDQ-D-20 total score than males. However, the differences in TAS-20 and subdomain score according to sex were not significant. Notably, TAS-20 and DDF (difficulty describing feelings) subdomain contributed to cognitive impairment in males, whereas both PHQ-9 total score and TAS-20 or DDF subdomain contributed to cognitive impairment in females.
Conclusion
These findings revealed significant sex differences in cognitive impairment and clinical correlates in SD, which should be further followed-up in the future.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13293-023-00488-w.
Keywords: Subthreshold depression, Depressive symptoms, Cognitive impairment, Alexithymia, Sex differences
Highlights
This study is the first to explore sex differences in subjective cognitive impairment and clinical associated risk factors in Chinese patients with subthreshold depression.
Notably, TAS-20 and DDF (difficulty describing feelings) subdomain contributed to cognitive impairment in males, whereas both PHQ-9 total score and TAS-20 or DDF subdomain contributed to cognitive impairment in females.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13293-023-00488-w.
Introduction
In recent decades, subthreshold depression (SD) has gained considerable attention due to its high prevalence in the population. Thus far, different terms have been used to describe this condition, such as subclinical, subsyndromal, or minor depression; however, SD refers to individuals with clinically relevant depressive symptoms that fall short of the criteria for a major depressive disorder (MDD) [1]. MDD is diagnosed when there are at least 5 out of 9 criteria symptoms for depression lasting minimally for 2 weeks, 1 of which is depressed mood or anhedonia. In comparison, SD is diagnosed when 2–4 criteria symptoms for depression and 1 core symptom, such as depressed mood or anhedonia are present for 2 weeks or longer [1, 2]. Even though SD is characterized by less severe symptoms than MDD, its health service utilization is greater than MDD on a population basis. Zhang et al. [3] conducted a meta-analysis of data from 113 studies covering 1,129,969 individuals, finding a summary prevalence of 11.02%. The prevalence in the youth group (aged < 18), the adult group (aged 18–60), and the elderly group (aged > 60) was 14.17%, 8.92%, and 12.95%, respectively. Moreover, individuals with SD are more vulnerable to developing MDD [3, 4]. Previous studies have shown that individuals with SD report moderate functional impairment, have poorer quality of life [5, 6], and tend to cope with enormous economic costs because of disability days [7].
Cognitive impairment is one of the major characteristics of patients suffering from depression. Accumulating evidence shows that cognitive symptoms in depressed patients lower their physical and mental efficiency. In addition, cognitive symptoms are commonly reported in both the acute phase and the remission of depressive symptoms. A large cross-sectional study conducted in six Asian countries showed that approximately 67.4% of medication-free outpatients with depression suffered from subjective memory deficits, and 73.2% suffered from subjective concentration deficits. It has been reported that subjective and objective cognition impairments further contribute to disability in patients with depression [8]. In recent decades, many studies have focused on the impact of cognitive symptoms such as slow thinking, lack of concentration, distractibility, memory problem, and decision-making difficulty on functioning [9]. For example, McIntyre et al. have reported that subjective cognitive dysfunction contributed more to poor workplace performance than a depressive symptom. However, far less attention has been paid to cognitive deficits in patients with SD. Hwang et al. [10] found that SD was associated with impaired resting-state functional connectivity of the cognitive control network, which is involved in cognitive processing (memory impairment, difficulties in decision making, and cognitive inflexibility) and cognitive biases (negative thoughts). Another study reported that implicit emotional neurocognitive processing was impaired in college students with SD.
Previous studies have found that women had a higher prevalence of SD than males [11, 12]. The meta-analysis showed that the estimates of SD in females (13.8%) were significantly higher than in males (9.68%) [3], which was similar to MDD [13]. Bennett et al. found gender differences in types of symptoms among depressed patients, with females being more likely to experience more depressed mood, appetite, and sleeping problems than males [14]. Another research found that girls exhibited more depressive mood and sleeping problems, whereas boys displayed higher levels of anhedonia, concentration problems, and psychomotor dysregulation. Also, girls performed worse than boys on variables such as social problem-solving and emotion regulation [15]. However, sex differences in cognitive deficits in patients with SD remained unclear.
Gaining a better understanding of sex differences in cognitive impairment in patients with SD is relevant, as it can affect treatment options and responses. In the present study, we aimed to explore sex differences in subjective cognitive impairment and clinically associated risk factors in Chinese patients with SD, which has not yet been assessed in patients with SD.
Methods
Subjects
This cross-sectional observational study was conducted at the psychological consultation clinic and outpatient service of Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai city, China, between November 1, 2021, and January 31, 2022. This study was approved by the Ethics committee of the Shanghai Mental Health Center (2021-49).
Convenience sampling methods were employed, and a QR code was distributed to collect all the information. Online psychological assessments included general information, alexithymia assessments, subjective cognitive assessment, and psychiatric symptom assessments (depression, anxiety). General information related to sex, age, education level, career, marital status, and history of smoking or drinking were collected. Participants were informed of the purpose of this study before assessments. All participants provided electronic informed consent. All items were set as required questions. Submitting a visiting serial number indicated the completion of the questionnaire.
Inclusion criteria were the following: (1) age between 18 and 45 years; (2) SD referred to a depressive state where patients had certain depression symptoms without meeting the criteria for major depression according to the International Classification of Diseases, 10th edition (ICD-10).
Exclusion criteria were: (1) those who met the ICD-10 criteria for MDD, mild depressive disorder or dysthymia; (2) major medical, neurological diseases; (3) substance dependence/abuse; (4) pregnancy or lactation; (5) with major psychotic disorder (schizophrenia, bipolar disorder).
Data collection and assessment
Depression symptoms were evaluated using the Patient Health Questionnaire depression (PHQ-9), which contained 9 items, each one ranging from 0 to 3. Depression Severity was ranked based on the total scores: normal (0–4), mild depression (5–9), moderate depression (10–14), moderate to severe depression (15–19), and severe depression (20 or greater). The Cronbach’s α coefficient of the Chinese version of the PHQ-9 and the test–retest reliability was reported to be 0.86 and 0.86, respectively. In the present study, Cronbach's α was 0.87, indicating a good internal consistency of these measurements.
The severity of anxiety was evaluated using the Generalized Anxiety Disorder-7 (GAD-7) scale, which contained 7 items, each one ranging from 0 to 3. Anxiety Severity was ranked based on the total scores, where 0–4 (no anxiety), 5–9 (mild anxiety), 10–14 (moderate anxiety), and 15–21 (severe anxiety). The Cronbach’s alpha coefficient of the Chinese version of the GAD-7 was 0.89, and the test–retest reliability was 0.85. In this study, Cronbach's α was 0.88, indicating a good internal consistency of these measurements.
Subjective cognitive deficits were evaluated using Perceived Deficits Questionnaire-Depression (PDQ-D), which included 20 items, and ranged from 0 (never in the past 7 days) to 4 (very often, more than once a day). The PDQ-D-20 evaluated four domains of cognitive function, including retrospective memory, attention/concentration, prospective memory, and planning/organization. Higher scores indicated a greater degree of cognition symptoms. The Chinese version of the PDQ-D-20 has been previously validated, revealing satisfactory reliability (Cronbach’s alpha coefficient = 0.948). In this study, Cronbach’s α was 0.9 which indicated a good internal consistency of these measurements.
In the present study, 20-item Toronto Alexithymia Scale (TAS-20) was used for assessing alexithymia. The TAS-20 consisted of three subscales for the subcomponents of alexithymia, including (1) difficulty identifying feelings and distinguishing them from bodily sensations of emotion (DIF), (2) difficulty describing feelings to others (DDF), and (3) externally oriented thinking (EOT). The TAS-20 included 20 items, where a score of each item ranged from 1 (totally disagree) to 5 (totally agree). Alexithymia severity was ranked based on the TAS-20 total scores, where ≤ 51 (no alexithymia), 52–60 (borderline alexithymia), and ≥ 61 (alexithymia). The TAS-20 has been validated and showed satisfactory reliability (Cronbach’s alpha coefficient ≥ 0.7). The Cronbach’s alpha coefficient of the Chinese version of the TAS-20 was 0.83, and the test–retest reliability was 0.87 [16].
Statistical analysis
SPSS version 25.0 (IBM SPSS Statistics for Macintosh, Armonk, NY, USA) was used to perform all the statistical analyses. The Kolmogorov–Smirnov test was used to test the normality of the distribution. All continuous data were expressed as the mean ± standard deviation (SD), while independent sample t-tests and Mann–Whitney U test was used for group comparisons; Chi-square test or Fisher’s exact test were employed for categorical variables. In addition, the correlation of PDQ-D-20 total score and clinical variables was assessed with partial correlation coefficients. Bonferroni corrections were used for multiple comparison corrections. Next, multiple linear regression analyses were conducted to identify characteristics related to cognitive impairment in all participants and the male and female groups separately. We also conducted multivariable regression analyses, where the PDQ-D-20 score was taken as the dependent variable. The following independent variables were entered into the model with the enter selection procedure: sex, age, GAD-7, PHQ-9, and TAS-20 total score. According to previous studies, these factors may have an impact on cognition [9, 17–20]. As the smoking rate differed between the male and female groups, smoking was controlled as a covariable. Sex by PHQ-9 and TAS-20 score interaction was added to the equation model. The sex subgroup was included in the equation with age, smoking, GAD-7, PHQ-9, and total score of TAS-20 as independent variables. We also performed multiple regression analyses as an exploratory approach considering the TAS-20 subscale (DIF, DDF, EOT) along with the same covariates in relation to subjective cognitive impairment. When there was collinearity between independent variables, the stepwise regression method was used. All statistical tests were two-sided, and p values < 0.05 were considered statistically significant.
Results
Social-demographic and clinical characteristics
Among a total of 200 subjects who were initially enrolled in the study, 23 participants did not complete cognitive function assessment (declaring it as meaningless or not finishing all processes), 20 patients were excluded due to age mismatch, and 28 patients refused to participate in the research (having no time or not being able to cooperate), resulting in 129 subjects who completed the whole assessments. In addition, a box-plot was performed, and 3 cases of PDQ-D-20 outliers were found. Finally, 126 people, 40 males and 86 females, were included in this study. In our study, the PDQ-D-20 total score of the patients was 32.03 ± 14.97, and their average age was 26.31 ± 7.22 years.
As shown in Table 1, the mean age in the male and female groups was 26.20 ± 6.63 years vs. 26.36 ± 7.52 years, respectively. Fewer female (2.3%) reported smoking compared to male (22.5%) (p = 0.001). The mean PDQ-D-20 total score of patients with SD in this study was 32.03 ± 14.97, being significantly higher in females than males (34.21 ± 15.00 vs. 27.35 ± 13.95) (F = 5.962, p = 0.016). Except for smoking and PDQ-D-20 total score, there were no significant differences in demographic and clinical characteristics between male and female patients. Therefore, smoking was controlled in the following analysis that compared sex differences in subjective cognitive impairment.
Table 1.
Social-demographic information and clinical characteristics of male and female patients with SD
| Variables | Total | Male (n = 40) | Female (n = 86) | t/χ2/Fisher | p value |
|---|---|---|---|---|---|
| Marital status | |||||
| No married | 87 | 28 (70.0%) | 59 (68.6%) | – | 0.728 |
| Married | 36 | 12 (30.0%) | 24 (27.9%) | ||
| Divorced/Widowed | 3 | – | 3 (3.5%) | ||
| Educational background | |||||
| High school or lower | 23 | 8 (20.0%) | 15 (17.4%) | 4.307 | 0.116 |
| College | 75 | 19 (47.5%) | 56 (65.1%) | ||
| Graduate or above | 28 | 13 (32.5%) | 15 (17.4%) | ||
| Career | |||||
| Worker | 6 | 4 (10.0%) | 2 (2.3%) | 4.217 | 0.239 |
| Staff | 55 | 18 (45.0%) | 37 (43.0%) | ||
| Student | 51 | 15 (37.5%) | 36 (41.9%) | ||
| Unemployed | 14 | 3 (7.5%) | 11 (12.8%) | ||
| Smoking | – | 0.001 | |||
| No | 115 | 31 (77.5%) | 84 (97.7%) | ||
| Yes | 11 | 9 (22.5%) | 2 (2.3%) | ||
| Drinking | – | 1.0 | |||
| No | 124 | 39 (97.5%) | 85 (98.8%) | ||
| Yes | 2 | 1 (2.5%) | 1 (1.2%) | ||
| Age | 26.20 ± 6.63 | 26.36 ± 7.52 | 0.116 | 0.908 | |
| GAD-7 score | 8.03 ± 5.62 | 7.71 ± 3.91 | 0.321 | 0.749 | |
| PHQ-9 score | 10.18 ± 4.03 | 9.99 ± 4.29 | 0.231 | 0.817 | |
| DIF | 20.05 ± 5.50 | 21.20 ± 5.60 | 1.076 | 0.284 | |
| DDF | 14.48 ± 2.72 | 15.00 ± 2.57 | 1.048 | 0.297 | |
| EOT | 26.13 ± 2.81 | 26.59 ± 3.21 | 0.791 | 0.430 | |
| TAS-20 score | 60.65 ± 7.62 | 62.79 ± 8.35 | − 1.376 | 0.171 | |
| PDQ-D-20 score | 27.35 ± 13.95 | 34.21 ± 15.00 | − 2.442 | 0.016 | |
GAD-7 Generalized Anxiety Disorder-7, PHQ-9 Patient Health Questionnaire depression-9, TAS-20 20-item Toronto Alexithymia Scale, PDQ-D-20 20-item Perceived Deficits Questionnaire-Depression, DIF Difficulty Identifying Feelings, DDF Difficulty Describing Feelings, EOT Externally Oriented Thinking
Bold values indicate statistical significance
Association between subjective cognition and clinical variables
For all patients, sex (β = 6.153, p = 0.025), TAS-20 score (β = 0.506, p = 0.001), and PHQ-9 score (β = 1.217, p < 0.001) were significantly associated with the multiple linear regression model (R2 = 0.260, adjusted R2 = 0.223, p < 0.001). The multivariable linear regression results are shown in Table 2. Furthermore, the sex*PHQ-9 and sex*TAS-20 score interaction terms were added in a multiple regression model with a stepwise selection procedure, revealing that sex*PHQ-9 (β = 0.632, t = 4.628, p < 0.001, 95% CI 0.362 to 0.902) and TAS-20 score (β = 0.506, t = 3.465, p = 0.001, 95% CI 0.217 to 0.796) were significantly associated with PDQ-D-20 score, and thus indicating that the relationship between PHQ-9 score and the subjective cognitive impairment differs between males and females. This interaction is depicted in Fig. 1.
Table 2.
Multiple linear regression results of PDQ-D-20 total score for all participants
| Independent variable | β | SE | β’ | t | p | β 95%CI |
|---|---|---|---|---|---|---|
| (Constant) | − 17.231 | 12.212 | − 1.411 | 0.161 | − 41.413, 6.951 | |
| Age | − 0.113 | 0.172 | − 0.054 | − 0.654 | 0.515 | − 0.453, 0.228 |
| Sex | 6.153 | 2.706 | 0.192 | 2.274 | 0.025 | 0.795, 11.510 |
| Smoking | 1.027 | 4.468 | 0.019 | 0.230 | 0.819 | − 7.821, 9.875 |
| GAD-7 score | − 0.240 | 0.298 | − 0.072 | − 0.805 | 0.423 | − 0.831, 0.351 |
| PHQ-9 score | 1.217 | 0.321 | 0.341 | 3.796 | < 0.001 | 0.582, 1.852 |
| TAS-20 score | 0.506 | 0.154 | 0.276 | 3.285 | 0.001 | 0.201, 0.811 |
GAD-7 generalized anxiety disorder-7, PHQ-9 Patient Health Questionnaire depression-9, TAS-20 20-item Toronto Alexithymia Scale, PDQ-D-20 20-item Perceived Deficits Questionnaire-Depression, CI confidence interval
Bold values indicated statistical significance
Fig. 1.
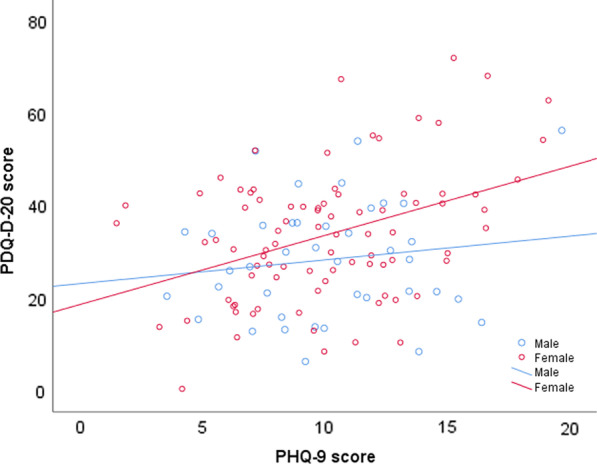
Relationship between 20-item Perceived Deficits Questionnaire-Depression (PDQ-D-20) score and Patient Health Questionnaire depression-9 (PHQ-9) score in males and females. Age, Generalized Anxiety Disorder-7 (GAD-7), 20-item Toronto Alexithymia Scale (TAS-20), and smoking were adjusted as covariates
We also conducted additional multiple regression analyses considering TAS-20 subscales (DIF, DDF, and EOT) along with the same covariates in relation to subjective cognitive impairment. When repeating the multiple linear regression analyses with TAS-20 subscore as independent variable, sex*PHQ-9 (β = 0.630, t = 4.688, p < 0.001, 95% CI 0.364–0.896) and DDF (β = 1.750, t = 3907, p < 0.001, 95% CI 0.863–2.637) were significantly associated with PDQ-D-20 score.
Sex difference in association of PDQ-D-20 with clinical variables
As shown in Table 3, after controlling for smoking, age, the PDQ-D-20 score was found to be related to the following characteristics in male patients: DIF (r = 0.321, df = 36, p = 0.049, PBonferroni = 0.294), DDF (r = 0.404, df = 36, p = 0.012, PBonferroni = 0.072), TAS-20 (r = 0.391, df = 36, p = 0.015, PBonferroni = 0.09); however, no variables passed Bonferroni correction (p > 0.05). In addition, multiple regression analysis with the stepwise procedure indicated that subjective cognitive impairment was significantly associated with TAS-20 (β = 0.705, t = 2.575, p = 0.014, adjusted R2 = 0.126) (Table 4). When repeating the linear regression analyses with the TAS-20 subscale as an independent variable, DDF (β = 2.137, t = 2.822, p = 0.008, adjusted R2 = 0.151) was significantly associated with PDQ-D-20 score in the male group. In the female group, subjective cognitive impairment was related to the following characteristics: PHQ-9 score (r = 0.385, df = 82, p < 0.001, PBonferroni < 0.01), DIF (r = 0.332, df = 82, p = 0.002, PBonferroni = 0.012), DDF (r = 0.379, df = 82, p < 0.001, PBonferroni < 0.01) and TAS-20 (r = 0.3337, df = 82, p = 0.002, PBonferroni = 0.012), all the characteristics persisted after Bonferroni correction (all p < 0.05, Table 3). Furthermore, multiple regression analysis with the stepwise procedure indicated that subjective cognition was significantly associated with PHQ-9 score (β = 1.301, t = 3.792, p < 0.001) and TAS-20 (β = 0.436, t = 2.473, p = 0.015), with adjusted R2 = 0.218 (Table 4). Also, the TAS-20 subscale was used as an independent variable, showing that the PHQ-9 score (β = 1.296, t = 3.842, p < 0.001) and DDF (β = 1.624, t = 2.884, p = 0.005) were significantly associated with PDQ-D-20 score in the female group, with adjusted R2 = 0.237 (Additional file 1: Table S1).
Table 3.
Correlations between PDQ-D-20 total score and clinical variables in male and female patients with SD
| Variables | Male (n = 40) | Female (n = 86) | ||
|---|---|---|---|---|
| r | p | r | p | |
| GAD-7 score | 0.165 | 0.323 | 0.080 | 0.468 |
| PHQ-9 score | 0.203 | 0.222 | 0.385 | < 0.001* |
| DIF | 0.321 | 0.049 | 0.332 | 0.002* |
| DDF | 0.404 | 0.012 | 0.379 | < 0.001* |
| EOT | 0.056 | 0.740 | − 0.008 | 0.944 |
| TAS-20 score | 0.391 | 0.015 | 0.337 | 0.002* |
GAD-7 generalized anxiety disorder-7, PHQ-9 Patient Health Questionnaire depression-9, TAS-20 20-item Toronto Alexithymia Scale, PDQ-D Perceived Deficits Questionnaire-Depression, DIF Difficulty Identifying Feelings, DDF Difficulty Describing Feeling, EOT Externally Oriented Thinking
Bold values indicated statistical significance
*After Bonferroni correction, statistical significance persisted
Table 4.
Clinical variables independently associated with PDQ-D-20 total score in male and female patients with SD
| Model | Independent variable | β | SE | β’ | t | p | β 95% CI | Adjusted R2 |
|---|---|---|---|---|---|---|---|---|
| Male (N = 40) | ||||||||
| 1 | (Constant) | − 15.424 | 16.740 | − 0.921 | 0.363 | − 49.313, 18.464 | 0.126 | |
| TAS-20 score | 0.705 | 0.274 | 0.385 | 2.575 | 0.014 | 0.151, 1.260 | ||
| Female (N = 86) | ||||||||
| 1 | (Constant) | 19.401 | 3.749 | 5.175 | < 0.001 | 11.945, 26.856 | 0.170 | |
| PHQ-9score | 1.483 | 0.345 | 0.424 | 4.296 | < 0.001 | 0.796, 2.169 | ||
| 2 | (Constant) | − 6.178 | 10.966 | − 0.563 | 0.575 | − 27.989, 15.633 | 0.218 | |
| PHQ-9 score | 1.301 | 0.343 | 0.372 | 3.792 | < 0.001 | 0.619, 1.983 | ||
| TAS-20 score | 0.436 | 0.176 | 0.243 | 2.473 | 0.015 | 0.085, 0.787 | ||
PHQ-9 Patient Health Questionnaire depression-9, PDQ-D-20 20-item Perceived Deficits Questionnaire-Depression, TAS-20 20-item Toronto Alexithymia Scale, SE standard error, CI confidence interval
Discussion
To the best of our knowledge, this is the first study that explored the sex differences in subjective cognitive impairment and its clinical correlates in Chinese patients with SD. The key findings of our research are: (a) there were no significant differences in the mean TAS-20 score, DIF, DDF, and EOT subconstructs between male and female groups; (b) female patients with SD had significantly higher PDQ-D-20 total score compared to male patients; (c) TAS-20 and DDF subdomain contributed to cognitive impairment in males, whereas both PHQ-9 total score and TAS-20 or DDF subdomain contributed to cognitive impairment in females.
While sex differences in the TAS-20 total score and the three subdomains have been extensively studied, the results remain inconsistent. We found no significant difference in overall and 3 subdomain scores between the two groups. For both student participants (n = 870) and psychological patients (n = 179) in the Chinese sample, sex differences on the overall TAS-20 score and the three subdomains score were not statistically significant [21]. However, other studies reported that men had significantly higher TAS-20 total scores than women in student or community samples [22–24]. Neumann et al. [25] reported that the TAS-20 total score, DDF, and EOT score were significantly higher in men than women participants. However, the DIF score did not differ between the two sex subgroups. Moreover, they found that age and education contributed to alexithymia [23]. Zhu et al. [24] surveyed medical students for alexithymia and found that male participants (n = 368) scored higher on the mean TAS-20 total score, DIF, DDF, and EOT compared with female participants (n = 1518). Another study reported that girls scored higher than boys on DIF subscales, and the TAS-20 total score did not differ between the two groups in Chinese adolescents [26], which might be due to some factors, such as sample size, different educational backgrounds, cultural differences, age or sex ratio disparity may explain the discrepant results. Therefore, further longitudinal studies with a larger sample are warranted to explore the sex differences of alexithymia in patients with SD.
Interestingly, the mean PDQ-D-20 total score of patients with SD in the present study was similar to the previous study [27], revealing the PDQ-D-20 total score of patients with MDD (30.3 ± 17.91), which was much higher than in community volunteers (9.28 ± 9.63). This finding indicated that patients with SD exhibited cognitive impairment. Furthermore, we found that three factors, i.e., sex, PHQ-9, and TAS-20, were independently associated with subjective cognitive impairment in patients with SD. Interaction between sex and PHQ-9 also contributed to subjective cognitive impairments. Our results showed sex differences in subjective cognitive impairment. Compared with male patients, females had more serious subjective cognitive impairment. It has been demonstrated that the relationship between depression and cognitive impairments varied by gender [28, 29]. Roh et al. reported that middle-aged and older women were more likely to display subjective cognitive impairment than men in the same age groups (OR = 1.59, 95% CI 1.46–1.73) [28]. In addition, Brown et al. [29] reported that the association between depression and subjective cognitive decline-related outcomes was regulated by age and gender. A recent study showed that subjective cognitive decline was more common in women than in men, and the score of cognitive complaints in the women group was significantly higher than in the men group [30]. The symptoms of subjective cognitive decline were assessed by subjective cognitive decline questionnaire-9 (SCD-Q9), where higher scores indicate more symptoms. Lin and his team [31] reported that the female factor contributes to the high SCD-Q9 score. However, another study reported conflicting results, detecting no significant gender differences in cognitive complaint [19]. Furthermore, no significant gender differences were found in the mean PDQ-D-20 total score across various levels of depression among full-time employees [32]. These discrepancies may be due to different diseases, sample representation, or research methods.
More importantly, there were sex differences in the relationship between subjective cognitive impairment and clinical variables of patients with SD. For males, cognitive impairment was significantly associated with the TAS-20 or DIF and DDF subdomain, while for females, cognitive impairment was significantly associated with the TAS-20 total score or the DIF and DDF subdomain, PHQ-9 score. Correlation analysis showed that the male group did not pass the Bonferroni correction. Yet, Bonferroni correction is a very conservative approach, which can easily incorrectly accept the null hypothesis. Notably, we first found that TAS-20 or DDF contributed to cognitive dysfunction in female and male patients with SD. Prior studies showed that alexithymia was correlated with cognitive dysfunction [33]. Galderisi et al. [33] reported that patients with panic disorder had a higher prevalence of alexithymia, lower verbal cognitive abilities, and difficulty inhibiting interference from nonverbal stimuli than the control group. In their study, Santorelli et al. reported that greater alexithymia and DDF were associated with poorer verbal executive function in older subjects (aged 61–92) but not in younger adults (aged 18–30) [20]. Correro et al. assessed executive functioning using neuropsychological testing and reported that alexithymia was significantly associated with age-related cognitive decline. Moreover, they found that high EOT contributed to poorer memory performance and DIF contributed to poor executive function in younger and older healthy adults [34].
Consistent with some previous studies [27, 35–37], we found that subjective cognitive impairment was significantly associated with the severity of depressive symptoms in the whole sample. In addition, Manit et al. [36] reported that PHQ-9 and PDQ-D were moderate to highly correlated (r = 0.69). However, one study reports did not find an association between cognitive impairment and the severity of depressive symptoms [38]. Nonetheless, this research did not explore sex differences in the relationship between subjective cognitive impairments and psychopathological symptoms. Interestingly, we found that the PHQ-9 score was associated with cognitive dysfunction in female patients but not in male patients, which was inconsistent with previous reports showing that the mean PDQ-D-20 score in men did not significantly differ from women across various levels of depression [32]. These discrepant results may be due to sampling representation, different stages of disease (acute episode vs. remission period), duration of illness, and exposure to antidepressant medication. For example, Galimberti et al. reported that a longer duration of untreated illness was associated with worse cognitive function during depression [39, 40].
The mechanisms of sex differences in the relationship between cognitive dysfunction and psychopathological symptoms remain unclear. Some factors, including social and psychological factors and biological factors, may contribute to sex differences. For example, gender differences in sex hormones [41] may contribute to varying degrees of cognitive impairment. In addition, the absence of the duration of the illness also contributed to bias. Unfortunately, as we did not examine the level of sex hormones or include this factor (duration of illness) in our study, further investigation is warranted to clarify the mechanism.
Limitations
There are several limitations in the present study. First, an important limitation is the absence of a healthy control group; hence, the reported findings should be interpreted with caution. Second, it is well known that cognitive symptoms are associated with some clinical variables, such as duration of illness, acute episode, or remission period. Unfortunately, we did not examine these factors. Therefore, further studies with large sample sizes and these relevant factors should be conducted to confirm these findings. Third, as it is difficult to guarantee the quality of the online survey, we only used a patient-reported questionnaire in this study, which could affect the accuracy of the data. Forth, due to the nature of the cross-sectional study design, causality between cognitive impairment and the associated risk factors cannot be inferred, which should be further investigated by future longitudinal studies.
Perspective and significance
The present study found sex differences in subjective cognitive impairment and a different association between subjective cognitive impairment and clinical correlates in females and males. Female patients had a higher PDQ-D-20 score than male patients, indicating worse cognitive impairment in females. Interestingly, subjective cognitive impairment was correlated with the TAS-20 and PHQ-9 scores in female patients, while only the TAS-20 score was in male patients. Therefore, improving the severity of depressive symptoms ameliorated cognitive dysfunction in females. Further studies should consider the sex role when assessing cognitive symptoms since its association with the clinical symptoms was distinct between males and females. In addition, further follow-up and controlled prospective studies with a large sample size could help clarify the interrelationship between cognitive impairment and clinical symptoms.
Supplementary Information
Additional file 1: Table S1. Clinical variables independently associated with PDQ-D-20 score in male and female patients with SD.
Acknowledgements
The authors thank all the investigators and subjects who participated in this study.
Author contributions
QYL participated in the literature search, data analysis, and data interpretation. QYL and XL wrote the manuscript, extracted, and collected data. QYL, XL, YZ, DFL, JJL, QFX, HL and YMW conceived of the study and participated in its design and coordination. CZW and ZHY participated in the study design and provided critical revision. All authors read and approved the final manuscript.
Funding
This work was supported by the STI 2030-Major Projects + 2022ZD0208500; Shanghai Science and Technology Innovation Action Plan Natural Science Fund Project (21ZR1455400); Shanghai Jiaotong University “Jiaotong University Star” Program Medical-Industrial Crossover Research Fund Project (YG2019QNB07); Jiangsu Province Science and Technology Plan Project Key R&D Program (BE2020661); CAS Key Laboratory of Mental Health, Institute of Psychology (KLMH2018K02); Liberal arts scientific research and innovation cultivation program of Shanghai Jiaotong University (WKCX1929); 2019 Genetic Development and Psychoneurological Disorders Ministry of Education Key Laboratory Open Course (2019GDND02).
Availability of data and materials
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were following the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qinyu lv and Xin Li have contributed equally to this work.
Contributor Information
Chongze Wang, Email: wangchongze@126.com.
Zhenghui Yi, Email: yizhenghui1971@163.com.
References
- 1.Kroenke K. When and how to treat subthreshold depression. JAMA. 2017;317:702–704. doi: 10.1001/jama.2017.0233. [DOI] [PubMed] [Google Scholar]
- 2.Kroenke K. Minor depression: midway between major depression and euthymia. Ann Intern Med. 2006;144:528–530. doi: 10.7326/0003-4819-144-7-200604040-00013. [DOI] [PubMed] [Google Scholar]
- 3.Zhang R, Peng X, Song X, et al. The prevalence and risk of developing major depression among individuals with subthreshold depression in the general population. Psychol Med, 2022:1–10. [DOI] [PMC free article] [PubMed]
- 4.Fogel J, Eaton WW, Ford DE. Minor depression as a predictor of the first onset of major depressive disorder over a 15-year follow-up. Acta Psychiatr Scand. 2006;113:36–43. doi: 10.1111/j.1600-0447.2005.00654.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee YY, Stockings EA, Harris MG, et al. The risk of developing major depression among individuals with subthreshold depression: a systematic review and meta-analysis of longitudinal cohort studies. Psychol Med. 2019;49:92–102. doi: 10.1017/S0033291718000557. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez MR, Nuevo R, Chatterji S, et al. Definitions and factors associated with subthreshold depressive conditions: a systematic review. BMC Psychiatry. 2012;12:181. doi: 10.1186/1471-244X-12-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broadhead WE, Blazer DG, George LK, et al. Depression, disability days, and days lost from work in a prospective epidemiologic survey. JAMA. 1990;264:2524–2528. doi: 10.1001/jama.1990.03450190056028. [DOI] [PubMed] [Google Scholar]
- 8.Naismith SL, Longley WA, Scott EM, et al. Disability in major depression related to self-rated and objectively-measured cognitive deficits: a preliminary study. BMC Psychiatry. 2007;7:32. doi: 10.1186/1471-244X-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Culpepper L, Lam RW, McIntyre RS. Cognitive impairment in patients with depression: awareness, assessment, and management. J Clin Psychiatry. 2017;78:1383–1394. doi: 10.4088/JCP.tk16043ah5c. [DOI] [PubMed] [Google Scholar]
- 10.Hwang JW, Egorova N, Yang XQ, et al. Subthreshold depression is associated with impaired resting-state functional connectivity of the cognitive control network. Transl Psychiatry. 2015;5:e683. doi: 10.1038/tp.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balazs J, Miklosi M, Kereszteny A, et al. Adolescent subthreshold-depression and anxiety: psychopathology, functional impairment and increased suicide risk. J Child Psychol Psychiatry. 2013;54:670–677. doi: 10.1111/jcpp.12016. [DOI] [PubMed] [Google Scholar]
- 12.Sihvola E, Keski-Rahkonen A, Dick DM, et al. Minor depression in adolescence: phenomenology and clinical correlates. J Affect Disord. 2007;97:211–218. doi: 10.1016/j.jad.2006.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salk RH, Hyde JS, Abramson LY. Gender differences in depression in representative national samples: meta-analyses of diagnoses and symptoms. Psychol Bull. 2017;143:783–822. doi: 10.1037/bul0000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett DS, Ambrosini PJ, Kudes D, et al. Gender differences in adolescent depression: do symptoms differ for boys and girls? J Affect Disord. 2005;89:35–44. doi: 10.1016/j.jad.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 15.Crockett MA, Martinez V, Jimenez-Molina A. Subthreshold depression in adolescence: gender differences in prevalence, clinical features, and associated factors. J Affect Disord. 2020;272:269–276. doi: 10.1016/j.jad.2020.03.111. [DOI] [PubMed] [Google Scholar]
- 16.Yi J, Yao S, Zhu X. The Chinese version of the tas-20: reliability and validity. Chin Ment Health J. 2003;17:763–767. [Google Scholar]
- 17.Smirni D, Beadle JN, Paradiso S. An initial study of alexithymia and its relationship with cognitive abilities among mild cognitive impairment, mild Alzheimer’s disease, and healthy volunteers. J Nerv Ment Dis. 2018;206:628–636. doi: 10.1097/NMD.0000000000000853. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Dong Q, Lu X, et al. Influence of comorbid anxiety symptoms on cognitive deficits in patients with major depressive disorder. J Affect Disord. 2020;260:91–96. doi: 10.1016/j.jad.2019.08.091. [DOI] [PubMed] [Google Scholar]
- 19.Overton M, Pihlsgard M, Elmstahl S. Prevalence and incidence of mild cognitive impairment across subtypes, age, and sex. Dement Geriatr Cogn Disord. 2019;47:219–232. doi: 10.1159/000499763. [DOI] [PubMed] [Google Scholar]
- 20.Santorelli GD, Ready RE. Alexithymia and executive function in younger and older adults. Clin Neuropsychol. 2015;29:938–955. doi: 10.1080/13854046.2015.1123296. [DOI] [PubMed] [Google Scholar]
- 21.Zhu X, Yi J, Yao S, et al. Cross-cultural validation of a Chinese translation of the 20-item Toronto alexithymia scale. Compr Psychiatry. 2007;48:489–496. doi: 10.1016/j.comppsych.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Parker JD, Taylor GJ, Bagby RM. The 20-item toronto alexithymia scale. Iii. Reliability and factorial validity in a community population. J Psychosom Res. 2003;55:269–275. doi: 10.1016/S0022-3999(02)00578-0. [DOI] [PubMed] [Google Scholar]
- 23.Lane RD, Sechrest L, Riedel R. Sociodemographic correlates of alexithymia. Compr Psychiatry. 1998;39:377–385. doi: 10.1016/S0010-440X(98)90051-7. [DOI] [PubMed] [Google Scholar]
- 24.Zhu Y, Luo T, Liu J, et al. Influencing factors of alexithymia in Chinese medical students: a cross-sectional study. BMC Med Educ. 2017;17:66. doi: 10.1186/s12909-017-0901-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neumann D, Zupan B. Sex differences in emotional insight after traumatic brain injury. Arch Phys Med Rehabil. 2020;101:1922–1928. doi: 10.1016/j.apmr.2020.04.018. [DOI] [PubMed] [Google Scholar]
- 26.Ling Y, Zeng Y, Yuan H, et al. Cross-cultural validation of the 20-item Toronto alexithymia scale in Chinese adolescents. J Psychiatr Ment Health Nurs. 2016;23:179–187. doi: 10.1111/jpm.12298. [DOI] [PubMed] [Google Scholar]
- 27.Shi C, Wang G, Tian F, et al. Reliability and validity of Chinese version of perceived deficits questionnaire for depression in patients with MDD. Psychiatry Res. 2017;252:319–324. doi: 10.1016/j.psychres.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 28.Roh M, Dan H, Kim O. Influencing factors of subjective cognitive impairment in middle-aged and older adults. Int J Environ Res Public Health. 2021;18:11488. doi: 10.3390/ijerph182111488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown MJ, Hill NL, Haider MR. Age and gender disparities in depression and subjective cognitive decline-related outcomes. Aging Ment Health. 2022;26:48–55. doi: 10.1080/13607863.2020.1861214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giacomucci G, Mazzeo S, Padiglioni S, et al. Gender differences in cognitive reserve: implication for subjective cognitive decline in women. Neurol Sci. 2022;43:2499–2508. doi: 10.1007/s10072-021-05644-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin LH, Wang SB, Xu WQ, et al. Subjective cognitive decline symptoms and its association with socio-demographic characteristics and common chronic diseases in the southern Chinese older adults. BMC Public Health. 2022;22:127. doi: 10.1186/s12889-022-12522-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawrence C, Roy A, Harikrishnan V, et al. Association between severity of depression and self-perceived cognitive difficulties among full-time employees. Prim Care Companion CNS Disord, 2013,15 [DOI] [PMC free article] [PubMed]
- 33.Galderisi S, Mancuso F, Mucci A, et al. Alexithymia and cognitive dysfunctions in patients with panic disorder. Psychother Psychosom. 2008;77:182–188. doi: 10.1159/000119738. [DOI] [PubMed] [Google Scholar]
- 34.Correro AN, 2nd, Paitel ER, Byers SJ, et al. The role of alexithymia in memory and executive functioning across the lifespan. Cogn Emot. 2021;35:524–539. doi: 10.1080/02699931.2019.1659232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feldman L, Lapin B, Busch RM, et al. Evaluating subjective cognitive impairment in the adult epilepsy clinic: Effects of depression, number of antiepileptic medications, and seizure frequency. Epilepsy Behav. 2018;81:18–24. doi: 10.1016/j.yebeh.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Manit S, Yee Ming M, Yen Kuang Y, et al. Cognitive dysfunction in Asian patients with depression (CogDAD): a cross-sectional study. Clin Pract Epidemiol Ment Health. 2017;13:185–199. doi: 10.2174/1745017901713010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srisurapanont M, Mok YM, Yang YK, et al. Cognitive complaints and predictors of perceived cognitive dysfunction in adults with major depressive disorder: findings from the cognitive dysfunction in Asians with depression (CogDAD) study. J Affect Disord. 2018;232:237–242. doi: 10.1016/j.jad.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 38.Keilp JG, Madden SP, Gorlyn M, et al. The lack of meaningful association between depression severity measures and neurocognitive performance. J Affect Disord. 2018;241:164–172. doi: 10.1016/j.jad.2018.08.034. [DOI] [PubMed] [Google Scholar]
- 39.Galimberti C, Bosi MF, Volonte M, et al. Duration of untreated illness and depression severity are associated with cognitive impairment in mood disorders. Int J Psychiatry Clin Pract. 2020;24:227–235. doi: 10.1080/13651501.2020.1757116. [DOI] [PubMed] [Google Scholar]
- 40.Ambaw A, Desalegn GT. Magnitude and correlates of cognitive impairment among major depressive disorder patients in Addis Ababa: institution based cross-sectional study. BMC Res Notes. 2019;12:135. doi: 10.1186/s13104-019-4184-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamson DK, Roes MM, Galea LA. Sex hormones and cognition: neuroendocrine influences on memory and learning. Compr Physiol. 2016;6:1295–1337. doi: 10.1002/cphy.c150031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Clinical variables independently associated with PDQ-D-20 score in male and female patients with SD.
Data Availability Statement
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.


